19.1.1 About the possibilities to solve the cardiac inverse problem
As discussed in Chapter 7, no unique solution exists for the inverse problem. From clinical practice it is possible to make accurate ECG diagnoses in some diseases and to estimate other diseases with an acceptable probability. How can this discrepancy between theory and practice be explained?
 It was said in Chapter 7 that the inverse solution is impossible if measurements cannot be made inside the source and if no additional information about the nature of the source is available. There is, however, much knowledge of the electrophysiological behavior of the heart. This limits the degrees of freedom of the source and reduces the degree of uncertainty in reaching a diagnosis. The following are examples of these helpful constraints:
It was said in Chapter 7 that the inverse solution is impossible if measurements cannot be made inside the source and if no additional information about the nature of the source is available. There is, however, much knowledge of the electrophysiological behavior of the heart. This limits the degrees of freedom of the source and reduces the degree of uncertainty in reaching a diagnosis. The following are examples of these helpful constraints:
The size, location, and orientation of the heart are well known and their variabilities are limited.
The action impulse of individual muscle cells can be ap proximated as having only two electrophysiological sta tes: (re)polarization and depolarization.
Each muscle cell exhibits a specific form of activation; depolarization is followed by repolarization after ap proximately 0.2-0.4 seconds.
The atria and the ventricles form temporarily separate regions of activation.
The propagation velocity of the activation front in vari ous parts of the heart muscle is known.
The conduction system has a dominant effect on initiation of the activation front.
The relationship between muscle load and muscle hyper trophy is well understood.
There are a limited number of causes of muscular over load.
The electrophysiological effect of ischemia on heart muscle is known.
The location of ischemia or infarction is governed by the anatomy of the coronary arteries.
There are a limited number of congenital cardiac abnor malities.
 These anatomical and physiological constraints limit the degrees of freedom of the inverse solution and usually make it possible to obtain solutions. However, in most cases the cardiac diagnosis must be made more accurately. The diagnosis often needs to be verified or completely made with other diagnostic methods like auscultation, x-ray, coronary angiography, radiocardiographic imaging, clinical chemistry, ultrasound, and so on.
These anatomical and physiological constraints limit the degrees of freedom of the inverse solution and usually make it possible to obtain solutions. However, in most cases the cardiac diagnosis must be made more accurately. The diagnosis often needs to be verified or completely made with other diagnostic methods like auscultation, x-ray, coronary angiography, radiocardiographic imaging, clinical chemistry, ultrasound, and so on.
19.1.2 Bioelectric principles in ECG diagnosis
This discussion of ECG diagnosis is based on the following three principles:
 First, the propagating activation front is characterized by its resultant vector. This signal can be detected and estimated through the lead vector according to Equation 11.16 and Figure 11.6 in Chapter 11.
First, the propagating activation front is characterized by its resultant vector. This signal can be detected and estimated through the lead vector according to Equation 11.16 and Figure 11.6 in Chapter 11.
  | (11.6) |
 When the heart's electric activity is considered a vector, it is usually easier first to examine the path (trajectory) of the vector's tip (the vectorcardiogram). Then the signals in the 12-lead ECG may be regarded as projections of the electric heart vector on the respective lead vectors as a function of time (multiplied by the absolute value of the lead vector).
When the heart's electric activity is considered a vector, it is usually easier first to examine the path (trajectory) of the vector's tip (the vectorcardiogram). Then the signals in the 12-lead ECG may be regarded as projections of the electric heart vector on the respective lead vectors as a function of time (multiplied by the absolute value of the lead vector).
 Second, the sensitivity of the lead may be considered distributed according to lead field theory. In this case the propagating activation front contributes to the ECG signal of the lead according to Equation 11.30, namely
Second, the sensitivity of the lead may be considered distributed according to lead field theory. In this case the propagating activation front contributes to the ECG signal of the lead according to Equation 11.30, namely
  | (11.30) |
 In this formulation the dipole sources are not reduced to a single resultant dipole, but are considered as spatially distributed. Furthermore, the volume conductor inhomogeneities are taken into account.
In this formulation the dipole sources are not reduced to a single resultant dipole, but are considered as spatially distributed. Furthermore, the volume conductor inhomogeneities are taken into account.
 Third, the solid angle theorem offers substantial help for understanding the formation of the ECG signal, especially in the diagnosis of myocardial infarction (see Equation 11.7):
Third, the solid angle theorem offers substantial help for understanding the formation of the ECG signal, especially in the diagnosis of myocardial infarction (see Equation 11.7):
  | (11.7) |
 In arriving at Equation 11.7, one assumes the double layer sources to be uniform, but otherwise takes into account their spatial distribution. However, the volume conductor is assumed to be infinite in extent and uniform.
In arriving at Equation 11.7, one assumes the double layer sources to be uniform, but otherwise takes into account their spatial distribution. However, the volume conductor is assumed to be infinite in extent and uniform.
 In this chapter, the forward problem of ECG diagnosis is discussed. This leads to the solution of the inverse problem through the empirical approach, as mentioned in Section 7.5.4. The empirical approach is acceptable in this case, because the purpose of this chapter is to be illustrative only.
In this chapter, the forward problem of ECG diagnosis is discussed. This leads to the solution of the inverse problem through the empirical approach, as mentioned in Section 7.5.4. The empirical approach is acceptable in this case, because the purpose of this chapter is to be illustrative only.
19.2 THE APPLICATION AREAS OF ECG DIAGNOSIS
The main applications of the ECG to cardiological diagnosis include the following (see also Figure 19.1):
19.3 DETERMINATION OF THE ELECTRIC AXIS OF THE HEART
The concept of the electric axis of the heart usually denotes the average direction of the electric activity throughout ventricular (or sometimes atrial) activation. The term mean vector is frequently used instead of "electric axis." The direction of the electric axis may also denote the instantaneous direction of the electric heart vector. This is shown in vectorcardiography as a function of time.
19.4 CARDIAC RHYTHM DIAGNOSIS
19.4.1 Differentiating the P-, QRS- and T-waves
Because of the anatomical difference of the atria and the ventricles, their sequential activation, depolarization, and repolarization produce clearly differentiable deflections. This may be possible even when they do not follow one another in the correct sequence: P-QRS-T.
19.4.2 Supraventricular rhythms
Definition
Cardiac rhythms may be divided into two categories: supraventricular (above the ventricles) and ventricular rhythms.
Normal sinus rhythm
Normal sinus rhythm is the rhythm of a healthy normal heart, where the sinus node triggers the cardiac activation. This is easily diagnosed by noting that the three deflections, P-QRS-T, follow in this order and are differentiable. The sinus rhythm is normal if its frequency is between 60 and 100/min.<
Sinus bradycardia
A sinus rhythm of less than 60/min is called sinus bradycardia. This may be a consequence of increased vagal or parasympathetic tone.
Sinus tachycardia
A sinus rhythm of higher than 100/min is called sinus tachycardia. It occurs most often as a physiological response to physical exercise or psychical stress, but may also result from congestive heart failure.
Sinus arrhythmia
If the sinus rhythm is irregular such that the longest PP- or RR-interval exceeds the shortest interval by 0.16 s, the situation is called sinus arrhythmia. This situation is very common in all age groups. This arrhythmia is so common in young people that it is not considered a heart disease. One origin for the sinus arrhythmia may be the vagus nerve which mediates respiration as well as heart rhythm. The nerve is active during respiration and, through its effect on the sinus node, causes an increase in heart rate during inspiration and a decrease during expiration. The effect is particularly pronounced in children.
Nonsinus atrial rhythm
The origin of atrial contraction may be located somewhere else in the atria other than the sinus node. If it is located close to the AV node, the atrial depolarization occurs in a direction that is opposite the normal one. An obvious consequence is that in the ECG the P-wave has opposite polarity..
Wandering pacemaker
The origin of the atrial contraction may also vary or wander. Consequently, the P-waves will vary in polarity, and the PQ-interval will also vary.
Paroxysmal atrial tachycardia (PAT)
Paroxysmal atrial tachycardia (PAT) describes the condition when the P-waves are a result of a reentrant activation front (circus movement) in the atria, usually involving the AV node. This leads to a high rate of activation, usually between 160 and 220/min. In the ECG the P-wave is regularly followed by the QRS-complex. The isoelectric baseline may be seen between the T-wave and the next P-wave.
Atrial flutter
When the heart rate is sufficiently elevated so that the isoelectric interval between the end of T and beginning of P disappears, the arrhythmia is called atrial flutter. The origin is also believed to involve a reentrant atrial pathway. The frequency of these fluctuations is between 220 and 300/min. The AV-node and, thereafter, the ventricles are generally activated by every second or every third atrial impulse (2:1 or 3:1 heart block).
Atrial fibrillation
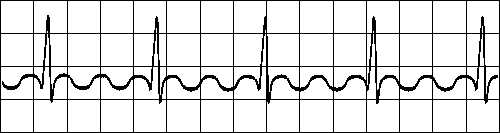
The activation in the atria may also be fully irregular and chaotic, producing irregular fluctuations in the baseline. A consequence is that the ventricular rate is rapid and irregular, though the QRS contour is usually normal. Atrial fibrillation occurs as a consequence of rheumatic disease, atherosclerotic disease, hyperthyroidism, and pericarditis. (It may also occur in healthy subjects as a result of strong sympathetic activation.)
Junctional rhythm
If the heart rate is slow (40-55/min), the QRS-complex is normal, the P-waves are possibly not seen, then the origin of the cardiac rhythm is in the AV node. Because the origin is in the juction between atria and ventricles, this is called junctional rhythm. Therefore, the activation of the atria occurs retrograde (i.e., in the opposite direction). Depending on whether the AV-nodal impulse reaches the atria before, simultaneously, or after the ventricles, an opposite polarity P-wave will be produced before, during, or after the QRS-complex, respectively. In the second case the P-wave will be superimposed on the QRS-complex and will not be seen.
19.4.3 Ventricular arrhythmias
Definition
In ventricular arrhythmias ventricular activation does not originate from the AV node and/or does not proceed in the ventricles in a normal way. If the activation proceeds to the ventricles along the conduction system, the inner walls of the ventricles are activated almost simultaneously and the activation front proceeds mainly radially toward the outer walls. As a result, the QRS-complex is of relatively short duration. If the ventricular conduction system is broken or the ventricular activation starts far from the AV node, it takes a longer time for the activation front to proceed throughout the ventricular mass.
Premature ventricular contraction
A premature ventricular contraction is one that occurs abnormally early. If its origin is in the atrium or in the AV node, it has a supraventricular origin. The complex produced by this supraventricular arrhythmia lasts less than 0.1 s. If the origin is in the ventricular muscle, the QRS-complex has a very abnormal form and lasts longer than 0.1 s. Usually the P-wave is not associated with it.
Idioventricular rhythm
If the ventricles are continuously activated by a ventricular focus whose rhythm is under 40/min, the rhythm is called idioventricular rhythm. The ventricular activity may also be formed from short (less than 20 s) bursts of ventricular activity at higher rates (between 40 and 120/min). This situation is called accelerated idioventricular rhythm.
Ventricular tachycardia
A rhythm of ventricular origin may also be a consequence of a slower conduction in ischemic ventricular muscle that leads to circular activation (re-entry). The result is activation of the ventricular muscle at a high rate (over 120/min), causing rapid, bizarre, and wide QRS-complexes; the arrythmia is called ventricular tachycardia. As noted, ventricular tachycardia is often a consequence of ischemia and myocardial infarction.
Ventricular fibrillation
When ventricular depolarization occurs chaotically, the situation is called ventricular fibrillation. This is reflected in the ECG, which demonstrates coarse irregular undulations without QRS-complexes. The cause of fibrillation is the establishment of multiple re-entry loops usually involving diseased heart muscle. In this arrhythmia the contraction of the ventricular muscle is also irregular and is ineffective at pumping blood. The lack of blood circulation leads to almost immediate loss of consciousness and death within minutes. The ventricular fibrillation may be stopped with an external defibrillator pulse and appropriate medication.
Pacer rhythm
A ventricular rhythm originating from a cardiac pacemaker is associated with wide QRS-complexes because the pacing electrode is (usually) located in the right ventricle and activation does not involve the conduction system. In pacer rhythm the ventricular contraction is usually preceded by a clearly visible pacer impulse spike. The pacer rhythm is usually set to 72/min..
19.5 DISORDERS IN THE ACTIVATION SEQUENCE
19.5.1 Atrioventricular conduction variations
Definition
As discussed earlier, if the P-waves always precede the QRS-complex with a PR-interval of 0.12-0.2 s, the AV conduction is normal and a sinus rhythm is diagnosed. If the PR-interval is fixed but shorter than normal, either the origin of the impulse is closer to the ventricles (see Section 19.4.2) or the atrioventricular conduction is utilizing an (abnormal) bypass tract leading to pre-excitation of the ventricles. The latter is called the Wolff-Parkinson-White syndrome and is discussed below. The PR-interval may also be variable, such as in a wandering atrial pacemaker and multifocal atrial tachycardia. Atrioventricular blocks are illustrated in Figure 19.4.
First-degree atrioventricular block
When the P-wave always precedes the QRS-complex but the PR-interval is prolonged over 0.2 s, first-degree atrioventricular block is diagnosed.
Second-degree atrioventricular block
If the PQ-interval is longer than normal and the QRS-complex sometimes does not follow the P-wave, the atrioventricular block is of second-degree. If the PR-interval progressively lengthens, leading finally to the dropout of a QRS-complex, the second degree block is called a Wenkebach phenomenon.
Third-degree atrioventricular block
Complete lack of synchronism between the P-wave and the QRS-complex is diagnosed as third-degree (or total) atrioventricular block. The conduction system defect in third degree AV-block may arise at different locations such as:
19.5.2 Bundle-branch block
Definition
Bundle-branch block denotes a conduction defect in either of the bundle-branches or in either fascicle of the left bundle-branch. If the two bundle-branches exhibit a block simultaneously, the progress of activation from the atria to the ventricles is completely inhibited; this is regarded as third-degree atrioventricular block (see the previous section). The consequence of left or right bundle-branch block is that activation of the ventricle must await initiation by the opposite ventricle. After this, activation proceeds entirely on a cell-to-cell basis. The absence of involvement of the conduction system, which initiates early activity of many sites, results in a much slower activation process along normal pathways. The consequence is manifest in bizarre shaped QRS-complexes of abnormally long duration. The ECG changes in connection with bundle- branch blocks are illustrated in Figure 19.5.
Right bundle-branch block
If the right bundle-branch is defective so that the electrical impulse cannot travel through it to the right ventricle, activation reaches the right ventricle by proceeding from the left ventricle. It then travels through the septal and right ventricular muscle mass. This progress is, of course, slower than that through the conduction system and leads to a QRS-complex wider than 0.1 s. Usually the duration criterion for the QRS-complex in right bundle-branch block (RBBB) as well as for the left brundle- branch block (LBBB) is >0.12 s.
Left bundle-branch block
The situation in left bundle-branch block (LBBB) is similar, but activation proceeds in a direction opposite to RBBB. Again the duration criterion for complete block is 0.12 s or more for the QRS-complex. Because the activation wavefront travels in more or less the normal direction in LBBB, the signals' polarities are generally normal. However, because of the abnormal sites of initiation of the left ventricular activation front and the presence of normal right ventricular activation the outcome is complex and the electric heart vector makes a slower and larger loop to the left and is seen as a broad and tall R-wave, usually in leads I, aVL, V5, or V6.
19.5.3 Wolff-Parkinson-White syndrome
One cause for a broad QRS-complex that exceeds over 0.12 s, may be the Wolff-Parkinson-White syndrome (WPW syndrome). In the WPW syndrome the QRS-complex initially exhibits an early upstroke called the delta wave. The interval from the P-wave to the R spike is normal, but the early ventricular excitation forming the delta wave shortens the PQ-time.
19.6 INCREASE IN WALL THICKNESS OR SIZE OF ATRIA AND VENTRICLES
19.6.1 Definition
Atrial and ventricular muscles react to physical stress in the same way as skeletal muscles: The muscles enlarge with increased amount of exercise. The extra tension may arise as a result of increased pressure load or volume load.
19.6.2 Atrial hypertrophy
Right atrial hypertrophy
Right atrial hypertrophy is a consequence of right atrial overload. This may be a result of tricuspid valve disease (stenosis or insufficiency), pulmonary valve disease, or pulmonary hypertension (increased pulmonary blood pressure). The latter is most commonly a consequence of chronic obstructive pulmonary disease or pulmonary emboli.
Left atrial hypertrophy
Left atrial hypertrophy is a consequence of left atrial overload. This may be a result of mitral valve disease (stenosis or insufficiency), aortic valve disease, or hypertension in the systemic circulation.
19.6.3 Ventricular hypertrophy
Right ventricular hypertrophy
Right ventricular hypertrophy is a consequence of right ventricular overload. This is caused by pulmonary valve stenosis, tricuspid insufficiency, or pulmonary hypertension (see above). Also many congenital cardiac abnormalities, such as a ventricular septal defect, may cause right ventricular overload.
Left ventricular hypertrophy
Left ventricular hypertrophy is a consequence of left ventricular overload. It arises from mitral valve disease, aortic valve disease, or systemic hypertension. Left ventricular hypertrophy may also be a consequence of obstructive hypertrophic cardiomyopathy, which is a sickness of the cardiac muscle cells.
19.7 MYOCARDIAL ISCHEMIA AND INFARCTION
If a coronary artery is occluded, the transport of oxygen to the cardiac muscle is decreased, causing an oxygen debt in the muscle, which is called ischemia. Ischemia causes changes in the resting potential and in the repolarization of the muscle cells, which is seen as changes in the T-wave. If the oxygen transport is terminated in a certain area, the heart muscle dies in that region. This is called an infarction. These are illustrated in Figure 19.8.
 Most of these application areas of ECG diagnosis are discussed in this chapter. Items 7, 8, and 9 - drug effect, electrolyte imbalance, and carditis - are not included in this discussion because their effects on the ECG signal cannot readily be explained with the methods included in this textbook.
Most of these application areas of ECG diagnosis are discussed in this chapter. Items 7, 8, and 9 - drug effect, electrolyte imbalance, and carditis - are not included in this discussion because their effects on the ECG signal cannot readily be explained with the methods included in this textbook.
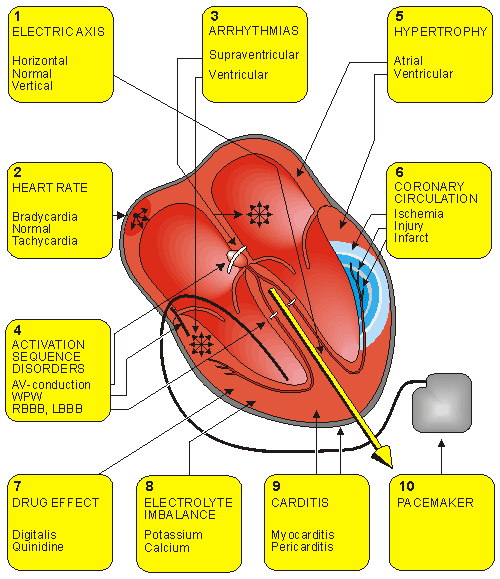
Fig. 19.1 Application areas of ECG diagnosis.
 The normal range of the electric axis lies between +30° and -110° in the frontal plane and between +30° and -30° in the transverse plane. (Note that the angles are given in the consistent coordinate system of the Appendix.)
The normal range of the electric axis lies between +30° and -110° in the frontal plane and between +30° and -30° in the transverse plane. (Note that the angles are given in the consistent coordinate system of the Appendix.)
 The direction of the electric axis may be approximated from the 12-lead ECG by finding the lead in the frontal plane, where the QRS-complex has largest positive deflection. The direction of the electric axis is in the direction of this lead vector. The result can be checked by observing that the QRS-complex is symmetrically biphasic in the lead that is normal to the electric axis. The directions of the leads were summarized in Figure 15.9. (In the evaluation of the ECG it is beneficial to use the lead -aVR instead of the lead aVR, as noted in Section 15.7.)
The direction of the electric axis may be approximated from the 12-lead ECG by finding the lead in the frontal plane, where the QRS-complex has largest positive deflection. The direction of the electric axis is in the direction of this lead vector. The result can be checked by observing that the QRS-complex is symmetrically biphasic in the lead that is normal to the electric axis. The directions of the leads were summarized in Figure 15.9. (In the evaluation of the ECG it is beneficial to use the lead -aVR instead of the lead aVR, as noted in Section 15.7.)
 Deviation of the electric axis to the right is an indication of increased electric activity in the right ventricle due to increased right ventricular mass. This is usually a consequence of chronic obstructive lung disease, pulmonary emboli, certain types of congenital heart disease, or other disorders causing severe pulmonary hypertension and cor pulmonale.
Deviation of the electric axis to the right is an indication of increased electric activity in the right ventricle due to increased right ventricular mass. This is usually a consequence of chronic obstructive lung disease, pulmonary emboli, certain types of congenital heart disease, or other disorders causing severe pulmonary hypertension and cor pulmonale.
 Deviation of the electric axis to the left is an indication of increased electric activity in the left ventricle due to increased left ventricular mass. This is usually a consequence of hypertension, aortic stenosis, ischemic heart disease, or some intraventricular conduction defect.
Deviation of the electric axis to the left is an indication of increased electric activity in the left ventricle due to increased left ventricular mass. This is usually a consequence of hypertension, aortic stenosis, ischemic heart disease, or some intraventricular conduction defect.
 The clinical meaning of the deviation of the heart's electric axis is discussed in greater detail in connection with ventricular hypertrophy.
The clinical meaning of the deviation of the heart's electric axis is discussed in greater detail in connection with ventricular hypertrophy.
 Identification of the normal QRS-complex from the P- and T-waves does not create difficulties because it has a characteristic waveform and dominating amplitude. This amplitude is about 1 mV in a normal heart and can be much greater in ventricular hypertrophy. The normal duration of the QRS is 0.08-0.09 s.
Identification of the normal QRS-complex from the P- and T-waves does not create difficulties because it has a characteristic waveform and dominating amplitude. This amplitude is about 1 mV in a normal heart and can be much greater in ventricular hypertrophy. The normal duration of the QRS is 0.08-0.09 s.
 If the heart does not exhibit atrial hypertrophy, the P-wave has an amplitude of about 0.1 mV and duration of 0.1 s. For the T-wave both of these numbers are about double. The T-wave can be differentiated from the P-wave by observing that the T-wave follows the QRS-complex after about 0.2 s.
If the heart does not exhibit atrial hypertrophy, the P-wave has an amplitude of about 0.1 mV and duration of 0.1 s. For the T-wave both of these numbers are about double. The T-wave can be differentiated from the P-wave by observing that the T-wave follows the QRS-complex after about 0.2 s.
 The origin of supraventricular rhythms (a single pulse or a continuous rhythm) is in the atria or AV junction, and the activation proceeds to the ventricles along the conduction system in a normal way. Supraventricular rhythms are illustrated in Figure 19.2.
The origin of supraventricular rhythms (a single pulse or a continuous rhythm) is in the atria or AV junction, and the activation proceeds to the ventricles along the conduction system in a normal way. Supraventricular rhythms are illustrated in Figure 19.2.
NORMAL SINUS RHYTHM
Impuses originate at S-A node at normal rate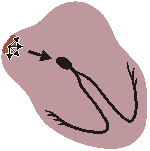

All complexes normal, evenly spaced
Rate 60 - 100/minFig. 19.2.A Normal sinus rhythm.
SINUS BRADYCARDIA
Impuses originate at S-A node at slow rate
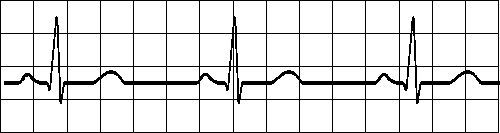
All complexes normal, evenly spaced
Rate < 60 - 100/minFig. 19.2.B Sinus bradycardia.
SINUS TACHYCARDIA
Impuses originate at S-A node at rapid rate
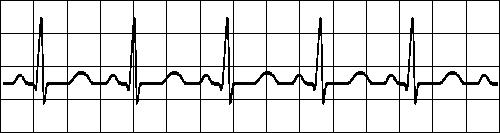
All complexes normal, evenly spaced
Rate > 100/minFig. 19.2.C Sinus tachycardia.
 Note, that in all of the preceding rhythms the length of the cardiac activation cycle (the P-QRS-T-waves together) is less than directly proportional to the PP-time. The main time interval change is between the T-wave and the next P-wave. This is easy to understand since the pulse rate of the sinus node is controlled mainly by factors external to the heart while the cardiac conduction velocity is controlled by conditions internal to the heart.
Note, that in all of the preceding rhythms the length of the cardiac activation cycle (the P-QRS-T-waves together) is less than directly proportional to the PP-time. The main time interval change is between the T-wave and the next P-wave. This is easy to understand since the pulse rate of the sinus node is controlled mainly by factors external to the heart while the cardiac conduction velocity is controlled by conditions internal to the heart.
SINUS TACHYCARDIA
Impuses originate at S-A node at rapid rate
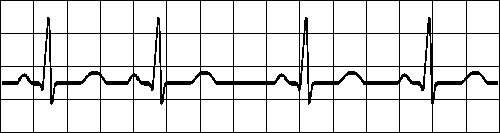
All complexes normal, rhythm is irregular
Longest R-R interval exceeds shirtest > 0.16 sFig. 19.2.D Sinus arrhythmia.
WANDERING PACEMAKER
Impuses originate from varying points in atria
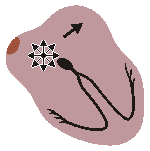
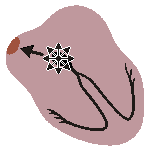
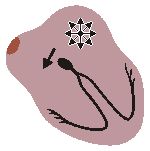
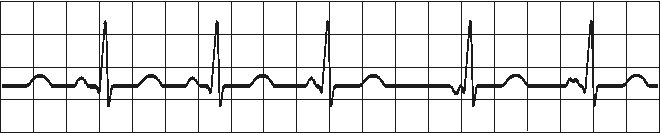
Variation in P-wave contour, P-R and P-P interval
and therefore in R-R intervalsFig. 19.2.E Wandering pacemaker.
ATRIAL FLUTTER
Impulses travel in circular course in atria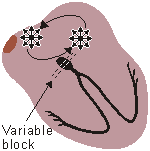
Rapid flutter waves, ventricular response irregular Fig. 19.2.F Atrial flutter.
ATRIAL FIBRILLATION
Impuses have chaotic, random pathways in atria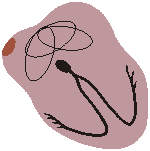
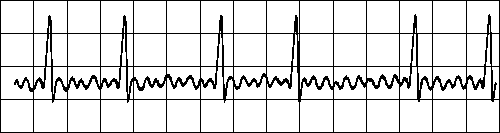
Baseline irregular, ventricular response irregular Fig. 19.2.G Atrial fibrillation.
JUNCTIONAL RHYTHM
Impuses originate at AV node with retrograde and antegrade direction
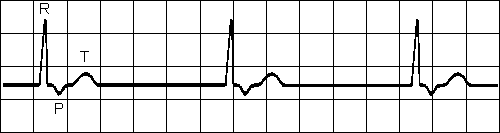
P-wave is often inverted, may be under or after QRS complex
Heart rate is slowFig. 19.2.H Junctional rhythm.
 The criterion for normal ventricular activation is a QRS-interval shorter than 0.1 s. A QRS-interval lasting longer than 0.1 s indicates abnormal ventricular activation. Ventricular arrhythmias are presented in Figure 19.3.
The criterion for normal ventricular activation is a QRS-interval shorter than 0.1 s. A QRS-interval lasting longer than 0.1 s indicates abnormal ventricular activation. Ventricular arrhythmias are presented in Figure 19.3.
PREMATURE VENTRICULAR CONTRACTION
A single impulse originates at right ventricle 
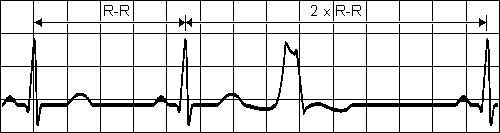
Time interval between normal R peaks
is a multiple of R-R intervalsFig. 19.3.A Premature ventricular contraction.
 The origin of the ventricular rhythm may be located by observing the polarity in various leads. The direction of the activation front is, of course, the direction of the lead vector in that lead where the deflection is most positive. The origin of the activation is, of course, on the opposite side of the heart when one is looking from this electrode.
The origin of the ventricular rhythm may be located by observing the polarity in various leads. The direction of the activation front is, of course, the direction of the lead vector in that lead where the deflection is most positive. The origin of the activation is, of course, on the opposite side of the heart when one is looking from this electrode.
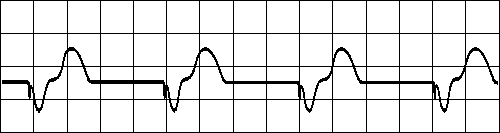
VENTRICULAR TACHYCARDIA
Impulse originate at ventricular pacemaker 
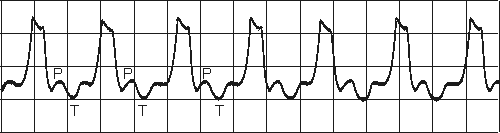
Wide ventricular complexes
Rate> 120/minFig. 19.3.B Ventricular tachycardia.
VENTRICULAR FIBRILLATION
Chaotic ventricular depolarization
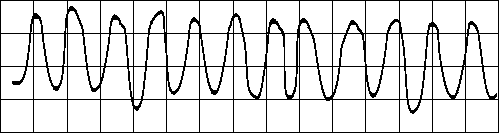
Rapid, wide, irregular ventricular complexes Fig. 19.3.C Ventricular fibrillation.
PACER RHYTHM
Impulses originate at transvenous pacemaker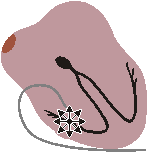
Rapid, wide, irregular ventricular complexes Fig. 19.3.D Pacer rhythm.
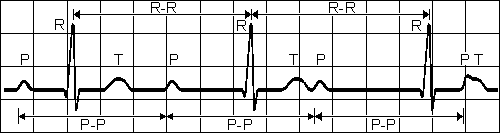
A-V BLOCK, FIRST DEGREE
Atrio-ventricular conduction lengthened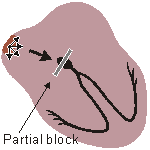

Rapid, wide, irregular ventricular complexes Fig. 19.4.A First-degree atrioventricular block.
A-V BLOCK, SECOND DEGREE
Sudden dropped QRS-complex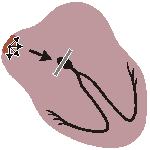
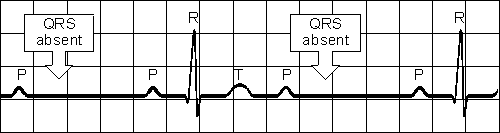
Intermittently skipped ventricular beat Fig. 19.4.B Second-degree atrioventricular block.
A-V BLOCK, THIRD DEGREE
Impulses originate at AV node and proceed to ventricles
Atrial and ventricular activities are not synchronous
P-P interval normal and constant,
QRS complexes normal, rate constant, 20 - 55 /minFig. 19.4.C Third-degree atrioventricular block.
 With normal activation the electrical forces of the right ventricle are partially concealed by the larger sources arising from the activation of the left ventricle. In right bundle-branch block (RBBB), activation of the right ventricle is so much delayed, that it can be seen following the activation of the left ventricle. (Activation of the left ventricle takes place normally.)
With normal activation the electrical forces of the right ventricle are partially concealed by the larger sources arising from the activation of the left ventricle. In right bundle-branch block (RBBB), activation of the right ventricle is so much delayed, that it can be seen following the activation of the left ventricle. (Activation of the left ventricle takes place normally.)
 RBBB causes an abnormal terminal QRS-vector that is directed to the right ventricle (i.e., rightward and anterior). This is seen in the ECG as a broad terminal S-wave in lead I. Another typical manifestation is seen in lead V1 as a double R-wave. This is named an RSR'-complex.
RBBB causes an abnormal terminal QRS-vector that is directed to the right ventricle (i.e., rightward and anterior). This is seen in the ECG as a broad terminal S-wave in lead I. Another typical manifestation is seen in lead V1 as a double R-wave. This is named an RSR'-complex.
RIGHT BUNDLE-BRANCH BLOCK
QRS duration greater than 0.12 s
Wide S wave in leads I, V5 and V6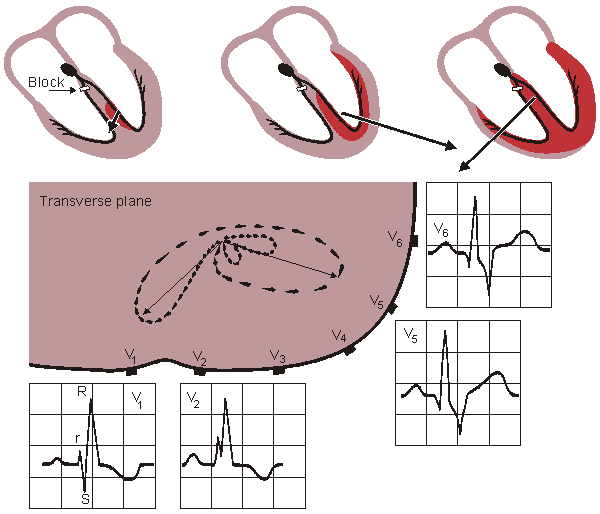
Fig. 19.5.A Right bundle-branch block.
LEFT BUNDLE-BRANCH BLOCK
QRS duration greater than 0.12 s
Wide S wave in leads V1 and V2, wide R wave in V5 and V6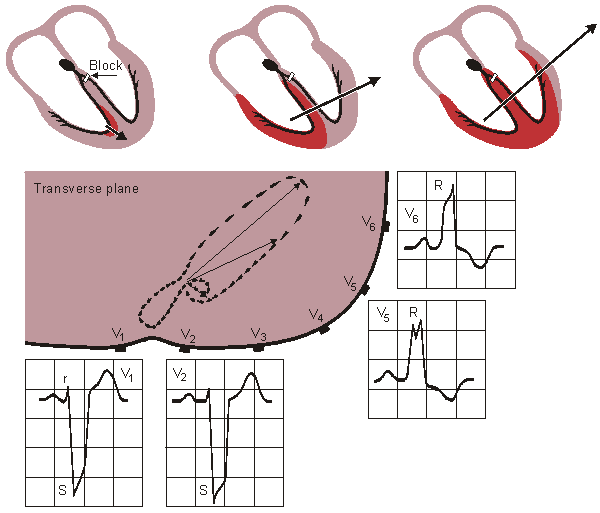
Fig. 19.5.B Left bundle-branch block.
 The cause of the WPW syndrome is the passage of activation from the atrium directly to the ventricular muscle via an abnormal route, called the bundle of Kent, which bypasses the AV junctions. This activates part of the ventricular muscle before normal activation reaches it via the conduction system (after a delay in the AV junction). The process is called pre-excitation, and the resulting ECG depends on the specific location of the accessory pathway.
The cause of the WPW syndrome is the passage of activation from the atrium directly to the ventricular muscle via an abnormal route, called the bundle of Kent, which bypasses the AV junctions. This activates part of the ventricular muscle before normal activation reaches it via the conduction system (after a delay in the AV junction). The process is called pre-excitation, and the resulting ECG depends on the specific location of the accessory pathway.
 Pressure overload is a consequence of increased resistance in the outflow tract of the particular compartment concerned (e.g., aortic stenosis). Volume overload means that either the outflow valve or the inflow valve of the compartment is incompetent, thus necessitating a larger stroke volume as compensation for the regurgitant backflow.
Pressure overload is a consequence of increased resistance in the outflow tract of the particular compartment concerned (e.g., aortic stenosis). Volume overload means that either the outflow valve or the inflow valve of the compartment is incompetent, thus necessitating a larger stroke volume as compensation for the regurgitant backflow.
 The increase in the atrial or ventricular size is called atrial or ventricular enlargement. The increase of the atrial or ventricular wall thickness is called atrial or ventricular hypertrophy. Very often they both are called hypertrophy, as in this presentation. Atrial and ventricular hypertrophies are illustrated in Figures 19.6 and 19.7, respectively.
The increase in the atrial or ventricular size is called atrial or ventricular enlargement. The increase of the atrial or ventricular wall thickness is called atrial or ventricular hypertrophy. Very often they both are called hypertrophy, as in this presentation. Atrial and ventricular hypertrophies are illustrated in Figures 19.6 and 19.7, respectively.
 In right atrial hypertrophy the electrical force due to the enlargened right atrium is larger. This electrical force is oriented mainly in the direction of lead II but also in leads aVF and III. In all of these leads an unusually large (i.e.,
In right atrial hypertrophy the electrical force due to the enlargened right atrium is larger. This electrical force is oriented mainly in the direction of lead II but also in leads aVF and III. In all of these leads an unusually large (i.e., 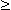 0.25 mV) P-wave is seen.
0.25 mV) P-wave is seen.
 In left atrial hypertrophy the electrical impulse due to the enlargened left atrium is strengthened. This electrical impulse is directed mainly along lead I or opposite to the direction of lead V1. Because the atrial activation starts from the right atrium, the aforementioned left atrial activation is seen later, and therefore, the P-wave includes two phases. In lead I these phases have the same polarities and in lead V1 the opposite polarities. This typical P-wave form is called the mitral P-wave. The specific diagnostic criterion for left atrial hypertrophy is the terminal portion of the P-wave in V1, having a duration
In left atrial hypertrophy the electrical impulse due to the enlargened left atrium is strengthened. This electrical impulse is directed mainly along lead I or opposite to the direction of lead V1. Because the atrial activation starts from the right atrium, the aforementioned left atrial activation is seen later, and therefore, the P-wave includes two phases. In lead I these phases have the same polarities and in lead V1 the opposite polarities. This typical P-wave form is called the mitral P-wave. The specific diagnostic criterion for left atrial hypertrophy is the terminal portion of the P-wave in V1, having a duration 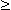 0.04 s and negative amplitude
0.04 s and negative amplitude 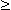 0.1 mV..
0.1 mV..
RIGHT ATRIAL HYPERTROPHY
Tall, peaked P wave in leads I and IILEFT ATRIAL HYPERTROPHY
Wide, notched P wave in lead II
Diphasic P wave in V1
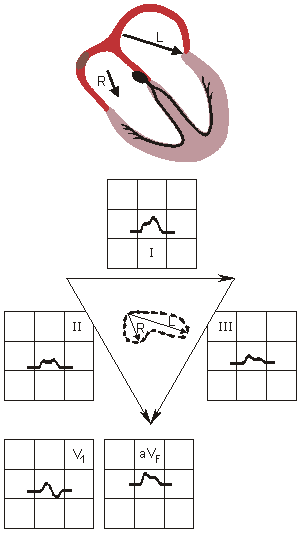
Fig. 19.6 Atrial hypertrophy.
 Right ventricular hypertrophy increases the ventricular electrical forces directed to the right ventricle - that is, to the right and front. This is seen in lead V1 as a tall R-wave of
Right ventricular hypertrophy increases the ventricular electrical forces directed to the right ventricle - that is, to the right and front. This is seen in lead V1 as a tall R-wave of  0.7 mV.
0.7 mV.
RIGHT VENTRICULAR HYPERTROPHY
Large R wave in leads V1 and V3
Large S wave in leads V6 and V6
Fig. 19.7.A Right ventricular hypertrophy.
 Left ventricular hypertrophy increases the ventricular electric forces directed to the left ventricle - that is, to the left and posteriorly. Evidence of this is seen in lead I as a tall R-wave and in lead III as a tall S-wave (
Left ventricular hypertrophy increases the ventricular electric forces directed to the left ventricle - that is, to the left and posteriorly. Evidence of this is seen in lead I as a tall R-wave and in lead III as a tall S-wave (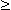 2.5 mV). Also a tall S-wave is seen in precordial leads V1 and V2 and a tall R-wave in leads V5 and V6, (
2.5 mV). Also a tall S-wave is seen in precordial leads V1 and V2 and a tall R-wave in leads V5 and V6, ( 3.5 mV).
3.5 mV).
LEFT VENTRICULAR HYPERTROPHY
Large S wave in leads V1 and V2
Large R wave in leads V6 and V6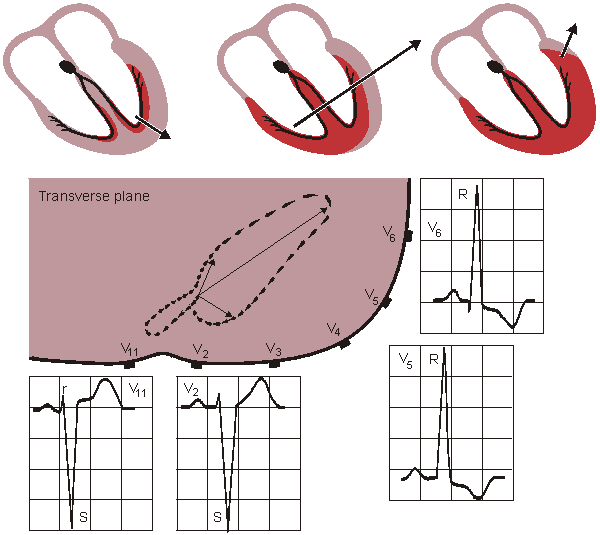
Fig. 19.7.B Left ventricular hypertrophy.
 An infarct area is electrically silent since it has lost its excitability. According to the solid angle theorem (Section 11.2.2) the loss of this outward dipole is equivalent to an electrical force pointing inward. With this principle it is possible to locate the infarction. (Of course, the infarct region also affects the activation sequence and the volume conductor so the outcome is more complicated.)
An infarct area is electrically silent since it has lost its excitability. According to the solid angle theorem (Section 11.2.2) the loss of this outward dipole is equivalent to an electrical force pointing inward. With this principle it is possible to locate the infarction. (Of course, the infarct region also affects the activation sequence and the volume conductor so the outcome is more complicated.)
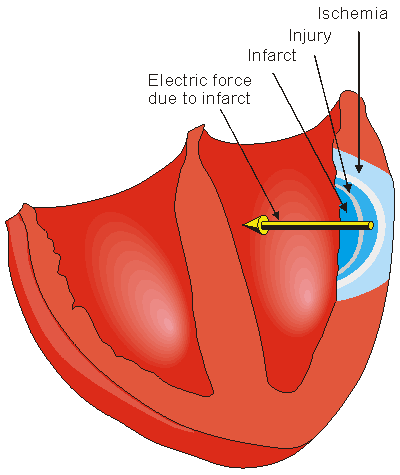
Figure 19.8 Myocardial ischemia and infarction.


