In previous chapters we described the need to take into account the interaction of the skin with electrodes whose purpose it was to record the surface potential noninvasively or to introduce stimulating currents. The skin and its properties were usually seen in these examples as providing certain difficulties to be understood and counteracted. In this chapter the sphere of interest is the skin response itself.
 Interest in the conductance between skin electrodes, usually placed at the palmar surface, arose because of the involvement of the sweat glands in this measurement. Since sweat gland activity, in turn, is controlled by sympathetic nerve activity, this measurement has been considered as an ideal way to monitor the autonomic nervous system. In this chapter we describe what is currently understood to underlie the electrodermal response (EDR) to sympathetic stimulation. The source of the material for this chapter comes mainly from the summary papers of Fowles (1974, 1986) and Venables and Christie (1980) which are suggested as the first recourse of the reader seeking further information.
Interest in the conductance between skin electrodes, usually placed at the palmar surface, arose because of the involvement of the sweat glands in this measurement. Since sweat gland activity, in turn, is controlled by sympathetic nerve activity, this measurement has been considered as an ideal way to monitor the autonomic nervous system. In this chapter we describe what is currently understood to underlie the electrodermal response (EDR) to sympathetic stimulation. The source of the material for this chapter comes mainly from the summary papers of Fowles (1974, 1986) and Venables and Christie (1980) which are suggested as the first recourse of the reader seeking further information.
 In the earlier chapters of this book such topics have been chosen that illustrate the fundamental principles of this discipline. In this chapter we discover that the basis for the EDR is not well understood and much remains to be discovered to explain the phenomena in basic physiological and biophysical terms. In spite of this shortcoming EDR is nevertheless widely used. Since it is a topic in bioelectricity it deserves attention precisely because of the need for further study. Clearly, here is a bioelectromagnetic application where a valid quantitative model would have an immediate and salutary effect on its use in research and in clinical applications.
In the earlier chapters of this book such topics have been chosen that illustrate the fundamental principles of this discipline. In this chapter we discover that the basis for the EDR is not well understood and much remains to be discovered to explain the phenomena in basic physiological and biophysical terms. In spite of this shortcoming EDR is nevertheless widely used. Since it is a topic in bioelectricity it deserves attention precisely because of the need for further study. Clearly, here is a bioelectromagnetic application where a valid quantitative model would have an immediate and salutary effect on its use in research and in clinical applications.
27.2 PHYSIOLOGY OF THE SKIN
The interpretation of skin conductance and/or skin potential requires some understanding about the structure of tissues at and beneath the skin surface. Figure 27.1 shows the main features of the skin. The most superficial layer is called the epidermis and consists of the stratum corneum, the stratum lucidum (seen only on "frictional surfaces"), the granular layer, the prickle cell layer, and the basal or germinating layer. The surface of the corneum (i.e., surface of the skin) is composed of dead cells, while at its base one finds healthy, living cells. Between these two sites there are transitional cells. This layer is also called the horny layer. Blood vessels are found in the dermis whereas the eccrine sweat gland secretory cells are found at the boundary between the dermis and the panniculus adiposus, also referred to as hypodermis and superficial fascia. The excretory duct of the eccrine sweat glands consists of a simple tube made up of a single or double layer of epithelial cells; this ascends to and opens on the surface of the skin. It is undulating in the dermis but then follows a spiral and inverted conical path through the epidermis to terminate in a pore on the skin surface. Cholinergic stimulation via fibers from the sympathetic nervous system constitutes the major influence on the production of sweat by these eccrine glands.
27.3 ELECTRODERMAL MEASURES
That the electrodermal response is associated with sweat gland activity is well established. Convincing evidence arises from experiments in which a direct correlation is seen between EDR and stimulated sweat gland activity. Furthermore, when sweat gland activity is abolished, then there is an absence of EDR signals (Fowles, 1986).
27.4 MEASUREMENT SITES AND CHARACTERISTIC SIGNALS
As discussed above, EDA is best measured at palmar sites. Suggested locations for electrode placement are given in Figure 27.2. In general, the electrodes used are of the Ag/AgCl type which are recessed from the skin and require the use of a suitable electrode paste. Since this is a reversible type of electrode, polarization and bias potentials are minimized. This is obviously of importance since such contributions introduce artifact in the SP and SC determinations. There is also a half-cell potential under each electrode, but if these are similar and overlie identical chloride concentrations their effects are equal and cancel. For this reason an electrode paste with NaCl at the concentration of sweat (approximately 0.3% NaCl) is to be preferred.
For constant-voltage conditions the voltage VA is measured across the series resistance. Then
Present-day practice utilizes a battery voltage Eb of 0.5 V, whereas constant current and constant voltage are better obtained electronically.
Fig. 27.3 (A) Upper trace is a slow-recovery SCR, whereas middle and lower are monophasic negative SPRs.
27.5 THEORY OF EDR
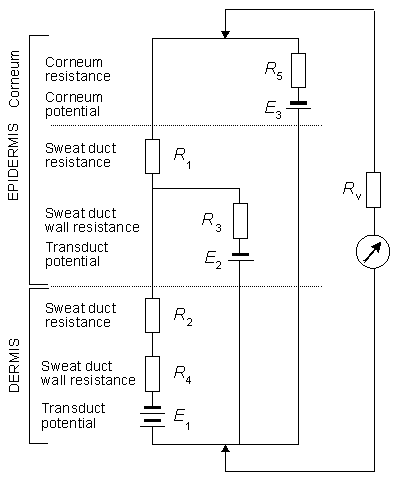
A comprehensive model underlying EDR has been developed by Fowles (1974) and appears essentially unchanged in Fowles (1986); its principle is given here in Figure 27.4. This model is useful only in a qualitative sense since there is no quantitative data either to support the circuit or to provide an evaluation of any of its elements. The top of the figure represents the surface of the skin, whereas the bottom represents the interface between the hypodermis and the dermis. The active electrode is at the top (skin surface), whereas the reference electrode is consired to be at the bottom (hypodermis).
Fig. 27.4 A simplified equivalent circuit describing the electrodermal system. Components are identified in the text. (From Fowles, 1986.)
27.6 APPLICATIONS
The applications of EDR lie in the area of psychophysiology and relate to studies in which a quantitative measure of sympathetic activity is desired. Fowles (1986) states:
 From an examination of Figure 27.1 one can appreciate that the epidermis ordinarily has a high electrical resistance due to the thick layer of dead cells with thickened keratin membranes. This aspect is not surprising, since the function of skin is to provide a barrier and protection against abrasion, mechanical assaults, and so on. The entire epidermis (with the exception of the desquamating cells) constitutes the barrier layer), a permeability barrier to flow. Experiments show its behavior to be that of a passive membrane.
From an examination of Figure 27.1 one can appreciate that the epidermis ordinarily has a high electrical resistance due to the thick layer of dead cells with thickened keratin membranes. This aspect is not surprising, since the function of skin is to provide a barrier and protection against abrasion, mechanical assaults, and so on. The entire epidermis (with the exception of the desquamating cells) constitutes the barrier layer), a permeability barrier to flow. Experiments show its behavior to be that of a passive membrane.
 However, the corneum is penetrated by the aforementioned sweat ducts from underlying cells; as these ducts fill, a relatively good conductor (sweat can be considered the equivalent of a 0.3% NaCl salt solution and, hence, a weak electrolyte) emerges, and many low-resistance parallel pathways result. A further increase in conductance results from the hydration of the corneum due to the flow of sweat across the duct walls (a process that is facilitated by the corkscrew duct pathway and the extremely hydrophilic nature of the corneum). As a consequence the effective skin conductance can vary greatly, depending on present and past eccrine activity. The aforementioned behavior is particularly great in the palmar and plantar regions because while the epidermis is very thick, at the same time the eccrine glands are unusually dense. It should be noted that the loading of ducts with sweat can be taking place before any (observable) release of sweat from the skin surface and/or noticeable diffusion into the corneum.
However, the corneum is penetrated by the aforementioned sweat ducts from underlying cells; as these ducts fill, a relatively good conductor (sweat can be considered the equivalent of a 0.3% NaCl salt solution and, hence, a weak electrolyte) emerges, and many low-resistance parallel pathways result. A further increase in conductance results from the hydration of the corneum due to the flow of sweat across the duct walls (a process that is facilitated by the corkscrew duct pathway and the extremely hydrophilic nature of the corneum). As a consequence the effective skin conductance can vary greatly, depending on present and past eccrine activity. The aforementioned behavior is particularly great in the palmar and plantar regions because while the epidermis is very thick, at the same time the eccrine glands are unusually dense. It should be noted that the loading of ducts with sweat can be taking place before any (observable) release of sweat from the skin surface and/or noticeable diffusion into the corneum.
 We have noted that the main function of the skin is to protect the body from the environment. One aspect of this is to prevent the loss of water by the body. However, at the same time, the evaporation of water as a means of regulating body temperature must be facilitated. These requirements appear to be carried out by the stratum corneum as a barrier layer that prevents the loss of water to the outside except through the sweat glands, whose activity can be controlled. This in turn is mediated by the autonomic (sympathetic) nervous system. Measurement of the output of the sweat glands, which EDR is thought to do, provides a simple gauge of the level and extent of sympathetic activity. This is the simple and basic concept underlying EDR and its application to psychophysiology.
We have noted that the main function of the skin is to protect the body from the environment. One aspect of this is to prevent the loss of water by the body. However, at the same time, the evaporation of water as a means of regulating body temperature must be facilitated. These requirements appear to be carried out by the stratum corneum as a barrier layer that prevents the loss of water to the outside except through the sweat glands, whose activity can be controlled. This in turn is mediated by the autonomic (sympathetic) nervous system. Measurement of the output of the sweat glands, which EDR is thought to do, provides a simple gauge of the level and extent of sympathetic activity. This is the simple and basic concept underlying EDR and its application to psychophysiology.
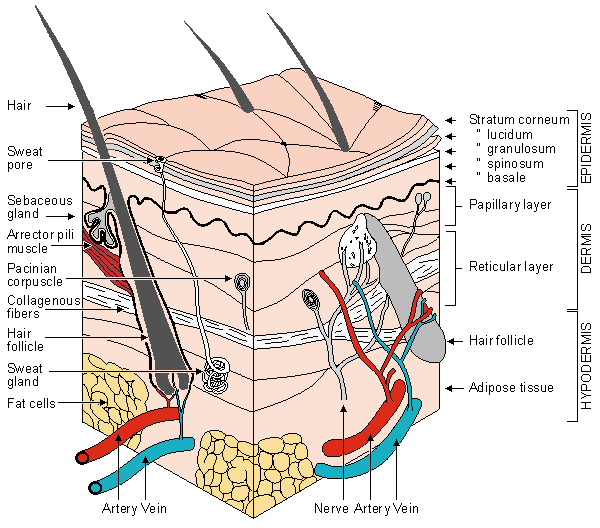
Fig. 27.1 Section of smooth skin taken from the sole of the foot. Blood vessels have been injected. (Redrawn from Ebling, Eady, and Leigh, 1992.)
 There are two major measures of the electrodermal response. The first, involving the measurement of resistance or conductance between two electrodes placed in the palmar region, was originally suggested by Féré (1888). It is possible also to detect voltages between these electrodes; these potential waveforms appear to be similar to the passive resistance changes, though its interpretation is less well understood. This measurement was pioneered by Tarchanoff (1889). The first type of measurement is referred to as exosomatic, since the current on which the measurement is based is introduced from the outside. The second type, which is less commonly used, is called endosomatic, since the source of voltage is internal. Researchers also distinguish whether the measurement is of the (tonic) background level (L), or the time-varying (phasic) response (R) type. These simple ideas have led to a number of specific measures, each described by a three letter-abbreviation. These are listed in Table 27.1.
There are two major measures of the electrodermal response. The first, involving the measurement of resistance or conductance between two electrodes placed in the palmar region, was originally suggested by Féré (1888). It is possible also to detect voltages between these electrodes; these potential waveforms appear to be similar to the passive resistance changes, though its interpretation is less well understood. This measurement was pioneered by Tarchanoff (1889). The first type of measurement is referred to as exosomatic, since the current on which the measurement is based is introduced from the outside. The second type, which is less commonly used, is called endosomatic, since the source of voltage is internal. Researchers also distinguish whether the measurement is of the (tonic) background level (L), or the time-varying (phasic) response (R) type. These simple ideas have led to a number of specific measures, each described by a three letter-abbreviation. These are listed in Table 27.1.
electrodermal measurements
Abbreviation Significance EDA Electrodermal Activity EDL Electrodermal Level EDR Electrodermal Response SCL Skin Conductance Level SCR Skin Conductance Response SRL Skin Resistance Level SRR Skin Resistance Response SPL Skin Potential Level SPR Skin Potential Response  Older terminology no longer in use, such as the galvanic skin response, has not been included in the table. The resistance and conductance measurements are reciprocals, of course; however, one or the other might turn out to be linearly related to the stimuli under study and be somewhat more useful as a result.
Older terminology no longer in use, such as the galvanic skin response, has not been included in the table. The resistance and conductance measurements are reciprocals, of course; however, one or the other might turn out to be linearly related to the stimuli under study and be somewhat more useful as a result.
 As described in Figure 27.2, the reference site should be abraded, a procedure that may possibly remove the corneum and introduce much reduced contact resistance. The site itself, on the forearm, is selected to be a neutral (nonactive) location so that only good contact is required. Although the removal of the corneum at the active site would interfere with the examination of the system there, no such requirement needs to be imposed at the reference site, since it should be nonactive.
As described in Figure 27.2, the reference site should be abraded, a procedure that may possibly remove the corneum and introduce much reduced contact resistance. The site itself, on the forearm, is selected to be a neutral (nonactive) location so that only good contact is required. Although the removal of the corneum at the active site would interfere with the examination of the system there, no such requirement needs to be imposed at the reference site, since it should be nonactive.
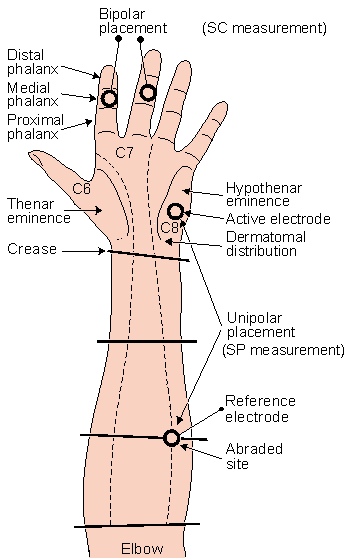
Fig. 27.2 Suggested electrode sites on the palm for the measurement of skin resistance and skin potentials. (Redrawn from Venables and Christie, 1980.)
 Shown in Figure 27.3 are signals characteristic of SCR and SPR waveforms. Those identified as having slow recovery, shown in Figure 27.3A, have a duration of around 40 s, with phasic amplitudes of around 2 µS for conductance and 10-20 mV for potential. Since the amplitude values depend on electrode area in a nonlinear way, these values cannot be readily normalized and, consequently, are difficult to compare with others. Data collected by Venables and Christie (1980) give a mean SCL of 0.3 µS and SCR of 0.52 µS in a study of a particular population (N = 500-600). Rapid-recovery SCRs and SPRs are shown in Figure 27.3B.
Shown in Figure 27.3 are signals characteristic of SCR and SPR waveforms. Those identified as having slow recovery, shown in Figure 27.3A, have a duration of around 40 s, with phasic amplitudes of around 2 µS for conductance and 10-20 mV for potential. Since the amplitude values depend on electrode area in a nonlinear way, these values cannot be readily normalized and, consequently, are difficult to compare with others. Data collected by Venables and Christie (1980) give a mean SCL of 0.3 µS and SCR of 0.52 µS in a study of a particular population (N = 500-600). Rapid-recovery SCRs and SPRs are shown in Figure 27.3B.
 The electronics associated with measurement of EDR is fairly simple. For exosomatic conditions either a constant current or a constant voltage source is used. As illustrated by Venables and Christie (1980), the circuit in either case consists of a battery with voltage EB connected to the skin through a series resistance RA; the circuit is completed by the skin resistance Rs. Constant current conditions can be implemented by letting RA be very large. (In the example given, EB = 100 V; RA = 10 MW; and, even for high values of skin resistance (i.e.,
The electronics associated with measurement of EDR is fairly simple. For exosomatic conditions either a constant current or a constant voltage source is used. As illustrated by Venables and Christie (1980), the circuit in either case consists of a battery with voltage EB connected to the skin through a series resistance RA; the circuit is completed by the skin resistance Rs. Constant current conditions can be implemented by letting RA be very large. (In the example given, EB = 100 V; RA = 10 MW; and, even for high values of skin resistance (i.e.,  , corresponding to 4 µS), the current differs from a nominal 10.0 µA by under 2.5%.) For constant-voltage conditions RA is small compared to Rs, so the voltage across Rs is the fixed battery voltage. In the constant-current case, the skin voltage Vs(t) is measured and
, corresponding to 4 µS), the current differs from a nominal 10.0 µA by under 2.5%.) For constant-voltage conditions RA is small compared to Rs, so the voltage across Rs is the fixed battery voltage. In the constant-current case, the skin voltage Vs(t) is measured and


(27.1)


(27.2)  For endosomatic measurements the skin potential is desired, and the optimum condition is where the input resistance of the amplifier is very high compared to the skin resistance. The use of an operational amplifier is called for. Additional requirements are evident from the sample waveforms in Figure 27.3: in general, an input voltage in the range of +10 to -70 mV at a bandwidth of from DC to a few Hz. Geddes and Baker (1989) suggest 0-5 Hz for tonic measurements, with 0.03-5 Hz being adequate for phasic measurements. Recommendations for electrodermal measurements were drawn up by a committee selected by the editor of Psychophysiology and published by that journal (Fowles et al., 1981). The paper by MacPherson, MacNeil, and Marble (1976) on measurement devices may also be useful.
For endosomatic measurements the skin potential is desired, and the optimum condition is where the input resistance of the amplifier is very high compared to the skin resistance. The use of an operational amplifier is called for. Additional requirements are evident from the sample waveforms in Figure 27.3: in general, an input voltage in the range of +10 to -70 mV at a bandwidth of from DC to a few Hz. Geddes and Baker (1989) suggest 0-5 Hz for tonic measurements, with 0.03-5 Hz being adequate for phasic measurements. Recommendations for electrodermal measurements were drawn up by a committee selected by the editor of Psychophysiology and published by that journal (Fowles et al., 1981). The paper by MacPherson, MacNeil, and Marble (1976) on measurement devices may also be useful.
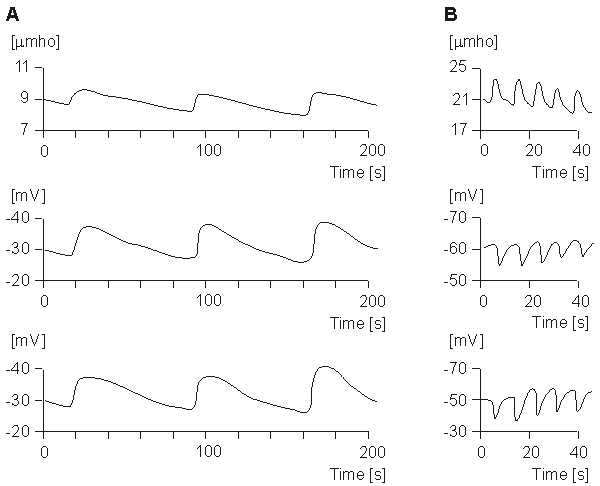
(B) The upper trace is a rapid-recovery SCR, whereas the middle and lower traces are positive monophasic SPRs. (Redrawn from Fowles, 1974.) R1 and R2 represent the resistance to current flow through the sweat ducts located in the epidermis and dermis, respectively. These are major current flow pathways when these ducts contain sweat, and their resistance decreases as the ducts fill. Such filling starts in the dermis and continues into the epidermis.
R1 and R2 represent the resistance to current flow through the sweat ducts located in the epidermis and dermis, respectively. These are major current flow pathways when these ducts contain sweat, and their resistance decreases as the ducts fill. Such filling starts in the dermis and continues into the epidermis.
 E1 and R4 represent access to the ducts through the duct wall in the dermis, whereas E2 and R3 describe the same pathway, but in the epidermis. Transduct potentials E1 and E2 arise as a result of unequal ionic concentrations across the duct as well as selective ionic permeabilities (as discussed in Chapter 3). This potential is affected by the production of sweat, particularly if, as is thought, the buildup of hydrostatic pressure results in depolarization of the ductal membranes. Such depolarization results in increased permeability to ion flow; this is manifested in the model by decreased values of R3 and R4. In particular, this is regarded as an important mechanism to explain rapid-recovery signals (since the restoration of normal permeability is equally fast). The potentials of E1 and E2 are normally lumen-negative.
E1 and R4 represent access to the ducts through the duct wall in the dermis, whereas E2 and R3 describe the same pathway, but in the epidermis. Transduct potentials E1 and E2 arise as a result of unequal ionic concentrations across the duct as well as selective ionic permeabilities (as discussed in Chapter 3). This potential is affected by the production of sweat, particularly if, as is thought, the buildup of hydrostatic pressure results in depolarization of the ductal membranes. Such depolarization results in increased permeability to ion flow; this is manifested in the model by decreased values of R3 and R4. In particular, this is regarded as an important mechanism to explain rapid-recovery signals (since the restoration of normal permeability is equally fast). The potentials of E1 and E2 are normally lumen-negative.
 The resistance R5 is that of the corneum, whereas E3 is its potential (treating this region as the site of liquid junction potentials). The phenomenon of hydration of the corneum, resulting from the diffusion of sweat from the sweat ducts into the normally dry and absorbant corneum, leads to a reduction in the value of R5.
The resistance R5 is that of the corneum, whereas E3 is its potential (treating this region as the site of liquid junction potentials). The phenomenon of hydration of the corneum, resulting from the diffusion of sweat from the sweat ducts into the normally dry and absorbant corneum, leads to a reduction in the value of R5.
 The predicted outcome of an experiment depends on (among others) the size of the response to a stimulus and the prior sweat gland condition. For an SCR determination Fowles (1986) states that the potentials can be ignored (these appear to be relatively small factors). If one assumes initial resting conditions, then a sweat response consists of sweat rising in the ducts, and correspondingly R2 slowly diminishes. The response latency is associated with the time required for this to take place. If the response is a small one and R1 and R5 are not affected, then the SCR may not show any change. For a larger response, although sweat still remains within the ducts, it now extends also into the corneum and hence reduces R1 as well as R2. If it is large enough, then flow across the duct wall will take place, causing hydration of the corneum and a decrease in R5. With a very large sweat response (or if a moderate response takes place after the ducts are already partly filled), then the response also includes the triggering of the epidermal duct membrane due to associated hydrostatic pressure buildup, and a consequent reduction of R3.
The predicted outcome of an experiment depends on (among others) the size of the response to a stimulus and the prior sweat gland condition. For an SCR determination Fowles (1986) states that the potentials can be ignored (these appear to be relatively small factors). If one assumes initial resting conditions, then a sweat response consists of sweat rising in the ducts, and correspondingly R2 slowly diminishes. The response latency is associated with the time required for this to take place. If the response is a small one and R1 and R5 are not affected, then the SCR may not show any change. For a larger response, although sweat still remains within the ducts, it now extends also into the corneum and hence reduces R1 as well as R2. If it is large enough, then flow across the duct wall will take place, causing hydration of the corneum and a decrease in R5. With a very large sweat response (or if a moderate response takes place after the ducts are already partly filled), then the response also includes the triggering of the epidermal duct membrane due to associated hydrostatic pressure buildup, and a consequent reduction of R3.
 For SP recordings Figure 27.4 can also serve as a guide on the possible outcome of the response to a stimulus. The measured potential is thought to represent, mainly, that across the epidermis - namely E3 minus the voltage drop in R5. Factors that are considered include the reabsorption of sodium across the duct walls by active transport which generates large lumen-negative potentials. Their effect on the measured potentials depends on the relative values of R1, R2, and R4 ((with low values enhancing surface measurement of E1, and low R5 values diminishing this measurement (Edelberg, 1968)). With modest responses when the corneum is relatively unhydrated, the increased lumen-negative duct potential and decrease in R2 and possibly R1 act to produce a monophasic negative SPR. Large responses that trigger the membrane response and a large and rapid decrease in R3 result in a decrease in the measured negative potential and possibly a positive component if the ducts are already filled.
For SP recordings Figure 27.4 can also serve as a guide on the possible outcome of the response to a stimulus. The measured potential is thought to represent, mainly, that across the epidermis - namely E3 minus the voltage drop in R5. Factors that are considered include the reabsorption of sodium across the duct walls by active transport which generates large lumen-negative potentials. Their effect on the measured potentials depends on the relative values of R1, R2, and R4 ((with low values enhancing surface measurement of E1, and low R5 values diminishing this measurement (Edelberg, 1968)). With modest responses when the corneum is relatively unhydrated, the increased lumen-negative duct potential and decrease in R2 and possibly R1 act to produce a monophasic negative SPR. Large responses that trigger the membrane response and a large and rapid decrease in R3 result in a decrease in the measured negative potential and possibly a positive component if the ducts are already filled.
 The reader can appreciate that the model is not a quantitative one and, hence, cannot be appealed to as a source of information regarding the outcome of an experiment except in very qualitative terms. One needs to examine to what extent a lumped- parameter circuit can represent the actual distributed system. Possibly such a circuit is justifiable; perhaps additional layers are needed. Most importantly, each circuit element needs to be described biophysically and quantitatively. Presumably this will require isolation of different parts of the system and also appropriate in vitro experiments. In the meantime, EDA appears to be useful as an empirical tool for registering the level of sympathetic activity in a psychophysiological experiment.
The reader can appreciate that the model is not a quantitative one and, hence, cannot be appealed to as a source of information regarding the outcome of an experiment except in very qualitative terms. One needs to examine to what extent a lumped- parameter circuit can represent the actual distributed system. Possibly such a circuit is justifiable; perhaps additional layers are needed. Most importantly, each circuit element needs to be described biophysically and quantitatively. Presumably this will require isolation of different parts of the system and also appropriate in vitro experiments. In the meantime, EDA appears to be useful as an empirical tool for registering the level of sympathetic activity in a psychophysiological experiment.
 One problem in the use of EDR should be mentioned. When skin conductance responses are used to evaluate an immediate outcome to a specific stimulus, it can be difficult to distinguish the stimulus specific response from the spontaneous SCR activity. To deal with this problem, investigators use a response window of 1-5 s following the stimulus, during which a signal will be accepted. If one assumes a spontaneous SCR rate of 7.5/min, the reduction in a confounding spontaneous SCR is 50%. A narrower window has been suggested to discriminate further against the unwanted signal.
One problem in the use of EDR should be mentioned. When skin conductance responses are used to evaluate an immediate outcome to a specific stimulus, it can be difficult to distinguish the stimulus specific response from the spontaneous SCR activity. To deal with this problem, investigators use a response window of 1-5 s following the stimulus, during which a signal will be accepted. If one assumes a spontaneous SCR rate of 7.5/min, the reduction in a confounding spontaneous SCR is 50%. A narrower window has been suggested to discriminate further against the unwanted signal.
 The stimuli that elicit these [EDA] responses are so ubiquitous that it has proved difficult to offer a conceptualization of the features common to these stimuli. There is no doubt, however, that the response often occurs to stimuli that depend for their efficacy on their physiological significance as opposed to their physical intensity.
The stimuli that elicit these [EDA] responses are so ubiquitous that it has proved difficult to offer a conceptualization of the features common to these stimuli. There is no doubt, however, that the response often occurs to stimuli that depend for their efficacy on their physiological significance as opposed to their physical intensity.  One measure of the extent of interest in EDR is the references to papers that list EDR as a keyword. In the SCI's Citation Index for 1991, one finds approximately 25 such references (i.e., publications). The importance attached to such measurements includes the statement in one recent paper that palmar sweat is one of the most salient symptoms of an anxiety state and, for some, the single most noticeable bodily reaction. But such applications lie outside the scope of this book, and we shall not pursue this topic further. The interested reader may wish to consult issues of the journal Psychophysiology for many of the current research papers.
One measure of the extent of interest in EDR is the references to papers that list EDR as a keyword. In the SCI's Citation Index for 1991, one finds approximately 25 such references (i.e., publications). The importance attached to such measurements includes the statement in one recent paper that palmar sweat is one of the most salient symptoms of an anxiety state and, for some, the single most noticeable bodily reaction. But such applications lie outside the scope of this book, and we shall not pursue this topic further. The interested reader may wish to consult issues of the journal Psychophysiology for many of the current research papers.


