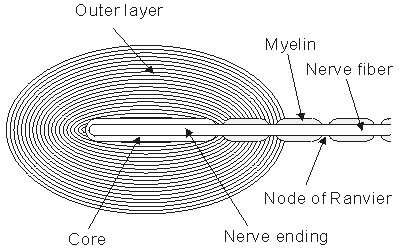
5.2 SYNAPSES
5.2.1 Structure and Function of the Synapse
The function of the synapse is to transfer electric activity (information) from one cell to another. The transfer can be from nerve to nerve (neuro-neuro), or nerve to muscle (neuro-myo). The region between the pre- and postsynaptic membrane is very narrow, only 30-50 nm. It is called the synaptic cleft (or synaptic gap). Direct electric communication between pre- and postjunctional cells does not take place; instead, a chemical mediator is utilized. The sequence of events is as follows:
- An action pulse reaches the terminal endings of the presynaptic cell.
- A neurotransmitter is released, which diffuses across the synaptic gap to bind to receptors in specialized membranes of the postsynaptic cell.
- The transmitter acts to open channels of one or several ion species, resulting in a change in the transmembrane potential. If depolarizing, it is an excitatory postsynaptic potential (EPSP); if hyperpolarizing, an inhibitory postsynaptic potential (IPSP).
Figure 5.1 shows the synapse between a nerve and muscle cell, a neuromuscular junction.
 In cardiac muscle the intercellular space between abutting cells is spanned by gap junctions, which provide a low-resistance path for the local circuit currents and may be regarded as an electric (myo-myo) synapse. (The gap, however, is not called a synaptic cleft.) This type of junction is discussed in a later chapter.
In cardiac muscle the intercellular space between abutting cells is spanned by gap junctions, which provide a low-resistance path for the local circuit currents and may be regarded as an electric (myo-myo) synapse. (The gap, however, is not called a synaptic cleft.) This type of junction is discussed in a later chapter.
 The presynaptic nerve fiber endings are generally enlarged to form terminal buttons or synaptic knobs. Inside these knobs are the vesicles that contain the chemical transmitters. The arrival of the action pulse opens voltage-gated Ca2+ channels that permit an influx of calcium ions. These in turn trigger the release into the synaptic gap, by exocytosis, of a number of the "prepackaged" vesicles containing the neurotransmitter.
The presynaptic nerve fiber endings are generally enlarged to form terminal buttons or synaptic knobs. Inside these knobs are the vesicles that contain the chemical transmitters. The arrival of the action pulse opens voltage-gated Ca2+ channels that permit an influx of calcium ions. These in turn trigger the release into the synaptic gap, by exocytosis, of a number of the "prepackaged" vesicles containing the neurotransmitter.
 On average, each neuron divides into perhaps 1000 synaptic endings. On the other hand, a single spinal motor neuron may have an average of 10,000 synaptic inputs. Based on this data, it is not surprising that the ratio of synapse to neurons in the human forebrain is estimated to be around 4×104. In neuro-neuro synapses, the postjunctional site may be a dendrite or cell body, but the former predominates.
On average, each neuron divides into perhaps 1000 synaptic endings. On the other hand, a single spinal motor neuron may have an average of 10,000 synaptic inputs. Based on this data, it is not surprising that the ratio of synapse to neurons in the human forebrain is estimated to be around 4×104. In neuro-neuro synapses, the postjunctional site may be a dendrite or cell body, but the former predominates.
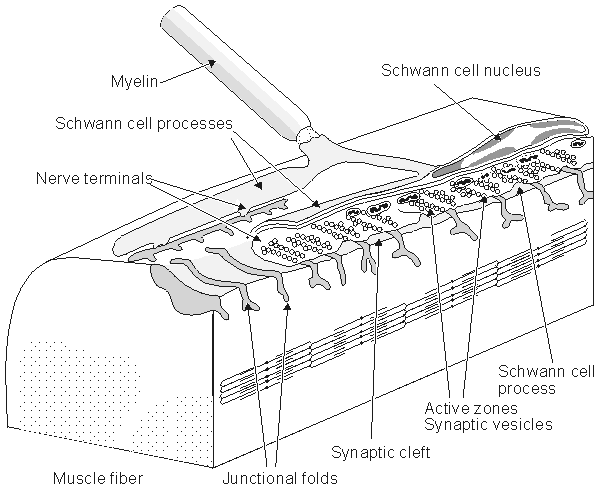
Fig. 5.1. The neuromuscular (synaptic) junction. Many features of this junction are also seen in the nerve-nerve synapse. The terminal ending of the prejunctional cell contains many vesicles, which are packages of the neurotransmitter acetylcholine (ACh). The gap between the pre- and postjunctional membrane is on the order of 15-30 nm. The transmitter is released by the arrival of an action impulse in the nerve; it diffuses and binds to receptors on the postjunctional muscle membrane, bringing about an EPSP and the initiation of a muscle action potential.
5.2.2 Excitatory and Inhibitory Synapses
In the neuromuscular junction, upon arrival of an action pulse at the motor neuron ending, acetylcholine (ACh) is released into the cleft. It diffuses across the gap to the muscle membrane where it binds to specialized receptors, resulting in a simultaneous increase in membrane permeability to both sodium and potassium ions. Because the relative effect on sodium exceeds that of potassium (described quantitatively later in this section), the membrane depolarizes and a postsynaptic action potential results. The process is always excitatory. Furthermore, arrival of a single action potential at the prejunctional site always results in sufficient release of transmitter to produce a transthreshold depolarization and initiate an action potential in the muscle.
 Synaptic inhibition occurs at nerve-nerve (neuro-neuro) junctions when presynaptic activity releases a transmitter that hyperpolarizes the postsynaptic membrane (i.e., makes its membrane voltage more negative). In theory, hyperpolarization could result from elevation of either potassium or chloride permeability because the equilibrium potential of each is more negative than the normal resting potential (which is influenced in the positive direction by the presence of sodium). In actuality, however, inhibition is due to elevated chloride permeability.
Synaptic inhibition occurs at nerve-nerve (neuro-neuro) junctions when presynaptic activity releases a transmitter that hyperpolarizes the postsynaptic membrane (i.e., makes its membrane voltage more negative). In theory, hyperpolarization could result from elevation of either potassium or chloride permeability because the equilibrium potential of each is more negative than the normal resting potential (which is influenced in the positive direction by the presence of sodium). In actuality, however, inhibition is due to elevated chloride permeability.
 In contrast with the neuromuscular (neuro-myo) junction, a single excitatory input to a neuro-neuro synapse is completely inadequate to depolarize the postjunctional membrane to threshold. In fact, with perhaps thousands of both excitatory and inhibitory inputs on the postjunctional cell, a spatial and temporal summation is continually taking place, and the membrane voltage will fluctuate. When, finally, a threshold of perhaps 10-15 mV is reached, an action potential results. In this way, an important integrative process takes place at the inputs to each nerve cell. The reader with computer science experience can appreciate the tremendous possibilities for information processing that can (and do!) take place, particularly when one considers that there are perhaps 1012 neurons and 1015 synapses in the human brain. This is indeed a neural net.
In contrast with the neuromuscular (neuro-myo) junction, a single excitatory input to a neuro-neuro synapse is completely inadequate to depolarize the postjunctional membrane to threshold. In fact, with perhaps thousands of both excitatory and inhibitory inputs on the postjunctional cell, a spatial and temporal summation is continually taking place, and the membrane voltage will fluctuate. When, finally, a threshold of perhaps 10-15 mV is reached, an action potential results. In this way, an important integrative process takes place at the inputs to each nerve cell. The reader with computer science experience can appreciate the tremendous possibilities for information processing that can (and do!) take place, particularly when one considers that there are perhaps 1012 neurons and 1015 synapses in the human brain. This is indeed a neural net.
 Presynaptic inhibition is another inhibition mechanism. In this case an inhibitory nerve ending (from another axon known as the presynaptic inhibitor) is synapsed to an excitatory presynaptic terminal. The inhibitory nerve releases a transmitter that partially depolarizes the presynaptic cell. As a consequence, activation arising in the presynaptic fiber is diminished, hence the release of transmitter is reduced. As a result, the degree of excitation produced in the postsynaptic cell is reduced (hence an inhibitory effect).
Presynaptic inhibition is another inhibition mechanism. In this case an inhibitory nerve ending (from another axon known as the presynaptic inhibitor) is synapsed to an excitatory presynaptic terminal. The inhibitory nerve releases a transmitter that partially depolarizes the presynaptic cell. As a consequence, activation arising in the presynaptic fiber is diminished, hence the release of transmitter is reduced. As a result, the degree of excitation produced in the postsynaptic cell is reduced (hence an inhibitory effect).
 The falling phase of the EPSP is characterized by a single time constant - that is, the time required for the response to a single excitatory stimulus to diminish to 1/e of its maximum. This is an important value. If a sequence of afferent stimuli occurs in a very short time interval, then temporal summation of the EPSPs occurs, yielding a growing potential. Similarly, if activity occurs at more than one synaptic knob simultaneously (or within the length of the aforementioned time constant), then spatial summation results. The additive effect on a synapse is nonlinear. Furthermore, the individual synapses interact in an extremely complicated way (Stevens, 1968). Despite these complexities, it has been shown experimentally that both spatial and temporal summation generally behave in a simple linear manner (Granit, Haase, and Rutledge, 1960; Granit and Renkin, 1961).
The falling phase of the EPSP is characterized by a single time constant - that is, the time required for the response to a single excitatory stimulus to diminish to 1/e of its maximum. This is an important value. If a sequence of afferent stimuli occurs in a very short time interval, then temporal summation of the EPSPs occurs, yielding a growing potential. Similarly, if activity occurs at more than one synaptic knob simultaneously (or within the length of the aforementioned time constant), then spatial summation results. The additive effect on a synapse is nonlinear. Furthermore, the individual synapses interact in an extremely complicated way (Stevens, 1968). Despite these complexities, it has been shown experimentally that both spatial and temporal summation generally behave in a simple linear manner (Granit, Haase, and Rutledge, 1960; Granit and Renkin, 1961).
 Synaptic transmission has been compared to an electric information transfer circuit in the following way: In the nerve axon the information is transferred by means of nerve impulses in "digital" or, more accurately, "pulse-code modulated" form. In the synapse, information is conducted with the transmitter substance in analog form, to be converted again in the next neuron into "digital" form. Though this analogy is not correct in all aspects, it illustrates the character of the neural information chain.
Synaptic transmission has been compared to an electric information transfer circuit in the following way: In the nerve axon the information is transferred by means of nerve impulses in "digital" or, more accurately, "pulse-code modulated" form. In the synapse, information is conducted with the transmitter substance in analog form, to be converted again in the next neuron into "digital" form. Though this analogy is not correct in all aspects, it illustrates the character of the neural information chain.
5.2.3 Reflex Arc
The driver of a car receives visual signals via photoreceptors that initiate coded afferent impulses that ascend nerve fibers and terminate in the visual cortex. Once the brain has processed the information, it sends efferent signals to the muscles in the foot and hands. Thus the car is slowed down and can make a right turn. But if our hand is mistakenly brought to rest on a hot surface, a set of signals to the hand and arm muscles result that are not initiated in the higher centers; cognition comes into play only after the fact. We say that a reflex path is involved in both of these examples. The first is complex and involves higher centers in the central nervous system, whereas the second describes a simpler reflex at a lower level. In fact, a great deal of reflex activity is taking place at all times of which we are unaware. For example, input signals are derived from internal sensors, such as blood pressure, or oxygen saturation in the blood, and so on, leading to an adjustment of heart rate, breathing rate, etc.
 The reflex arc, illustrated above, is considered to be the basic unit of integrated neural activity. It consists essentially of a sensory receptor, an afferent neuron, one or more synapses, an efferent neuron, and a muscle or other effector. The connection between afferent and efferent pathways is found, generally, in the spinal cord or the brain. The simplest reflex involves only a single synapse between afferent and efferent neurons (a monosynaptic reflex); an example is the familiar knee jerk reflex.
The reflex arc, illustrated above, is considered to be the basic unit of integrated neural activity. It consists essentially of a sensory receptor, an afferent neuron, one or more synapses, an efferent neuron, and a muscle or other effector. The connection between afferent and efferent pathways is found, generally, in the spinal cord or the brain. The simplest reflex involves only a single synapse between afferent and efferent neurons (a monosynaptic reflex); an example is the familiar knee jerk reflex.
 Homeostasis refers to the various regulatory processes in the body that maintain a normal state in the face of disturbances. The autonomic nervous system is organized to accomplish this automatically with regard to many organs of the body; its activity, like that of the somatic nervous system, is based on the reflex arc. In this case signals, which arise at visceral receptors, are conveyed via afferent neurons to the central nervous system, where integration takes place, resulting in efferent signals to visceral effectors (in particular, smooth muscle) to restore or maintain normal conditions. Integration of signals affecting blood pressure and respiration takes place in the medulla oblongata; those controlling pupillary response to light are integrated in the midbrain, whereas those responding to body temperature are integrated in the hypothalamus - to give only a few examples.
Homeostasis refers to the various regulatory processes in the body that maintain a normal state in the face of disturbances. The autonomic nervous system is organized to accomplish this automatically with regard to many organs of the body; its activity, like that of the somatic nervous system, is based on the reflex arc. In this case signals, which arise at visceral receptors, are conveyed via afferent neurons to the central nervous system, where integration takes place, resulting in efferent signals to visceral effectors (in particular, smooth muscle) to restore or maintain normal conditions. Integration of signals affecting blood pressure and respiration takes place in the medulla oblongata; those controlling pupillary response to light are integrated in the midbrain, whereas those responding to body temperature are integrated in the hypothalamus - to give only a few examples.
5.2.4 Electric Model of the Synapse
At the neuromuscular junction, Fatt and Katz (1951) showed that acetylcholine significantly increases the permeability of the cell membrane to small ions, whereas Takeuchi and Takeuchi (1960) demonstrated that chloride conductance was unaffected (in fact, gCl 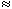 0). What happens if the membrane becomes equally permeable to sodium and potassium ions? Such a condition would alter the membrane potential from near the potassium Nernst potential to a value that approximates the average of the sodium and potassium equilibrium potentials. (This potential, in turn, is close to zero transmembrane voltage and is entirely adequate to initiate an activation.) If the postsynaptic region is voltage-clamped, the value that reduces the membrane current to zero during transmitter release is called the reversal voltage Vr. One can show that it equals the average Nernst potential of sodium and potassium, as mentioned above. In the neuromuscular junction in skeletal muscle, this reversal voltage is about -15 mV.
0). What happens if the membrane becomes equally permeable to sodium and potassium ions? Such a condition would alter the membrane potential from near the potassium Nernst potential to a value that approximates the average of the sodium and potassium equilibrium potentials. (This potential, in turn, is close to zero transmembrane voltage and is entirely adequate to initiate an activation.) If the postsynaptic region is voltage-clamped, the value that reduces the membrane current to zero during transmitter release is called the reversal voltage Vr. One can show that it equals the average Nernst potential of sodium and potassium, as mentioned above. In the neuromuscular junction in skeletal muscle, this reversal voltage is about -15 mV.
 The electric behavior at a synapse can be estimated by examining an equivalent circuit of the postsynaptic membrane, such as that shown in Figure 5.2. Two regions are identified: One represents the membrane associated with receptors sensitive to the transmitter, and the other the normal excitable membrane of the cell. In Figure 5.2 these two regions are represented by discrete elements, but in reality these are distributed along the structure that constitutes the actual cell. This figure depicts a neuromuscular junction, where the release of acetylcholine results in the elevation of sodium and potassium conductance in the target region, which is in turn depicted by the closing of the ACh switch. Upon closure of this switch,
The electric behavior at a synapse can be estimated by examining an equivalent circuit of the postsynaptic membrane, such as that shown in Figure 5.2. Two regions are identified: One represents the membrane associated with receptors sensitive to the transmitter, and the other the normal excitable membrane of the cell. In Figure 5.2 these two regions are represented by discrete elements, but in reality these are distributed along the structure that constitutes the actual cell. This figure depicts a neuromuscular junction, where the release of acetylcholine results in the elevation of sodium and potassium conductance in the target region, which is in turn depicted by the closing of the ACh switch. Upon closure of this switch,
 A discussion of the nervous system might logically begin with sensory cells located at the periphery of the body. These cells initiate and conduct signals to the brain and provide various sensory inputs such as vision, hearing, posture, and so on. Providing information on the environment to the body, these peripheral cells respond to stimuli with action pulses, which convey their information through encoded signals. These signals are conducted axonally through ascending pathways, across synapses, and finally to specific sites in the brain. Other neural cells in the brain process the coded signals, and direct the actions of muscles and other organs in response to the various sensory inputs. The entire circuit is recognized as a reflex arc, a basic unit in the nervous system. In some cases it is entirely automatic, and in others it is under voluntary control.
A discussion of the nervous system might logically begin with sensory cells located at the periphery of the body. These cells initiate and conduct signals to the brain and provide various sensory inputs such as vision, hearing, posture, and so on. Providing information on the environment to the body, these peripheral cells respond to stimuli with action pulses, which convey their information through encoded signals. These signals are conducted axonally through ascending pathways, across synapses, and finally to specific sites in the brain. Other neural cells in the brain process the coded signals, and direct the actions of muscles and other organs in response to the various sensory inputs. The entire circuit is recognized as a reflex arc, a basic unit in the nervous system. In some cases it is entirely automatic, and in others it is under voluntary control.
 No neurons run directly from the periphery to the brain. Normally the initiated signal is relayed by several intermediate neural cells. The interconnection between neurons, called the synapse, behaves as a simple switch but also has a special role in information processing. The junction (synapse) between a neural cell and the muscle that it innervates, called the neuromuscular junction, has been particularly well studied and provides much of our quantitative understanding about synapses. Since it is impossible to discuss the structure of the nervous system without including synapses, we begin our discussion with an examination of that topic.
No neurons run directly from the periphery to the brain. Normally the initiated signal is relayed by several intermediate neural cells. The interconnection between neurons, called the synapse, behaves as a simple switch but also has a special role in information processing. The junction (synapse) between a neural cell and the muscle that it innervates, called the neuromuscular junction, has been particularly well studied and provides much of our quantitative understanding about synapses. Since it is impossible to discuss the structure of the nervous system without including synapses, we begin our discussion with an examination of that topic.
 DINa = DGNa(Vm - VNa)
DINa = DGNa(Vm - VNa) DIK = DGK(Vm - VK)
DIK = DGK(Vm - VK)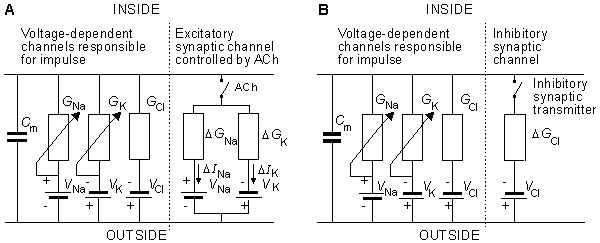




 0). What happens if the membrane becomes equally permeable to sodium and potassium ions? Such a condition would alter the membrane potential from near the potassium Nernst potential to a value that approximates the average of the sodium and potassium equilibrium potentials. (This potential, in turn, is close to zero transmembrane voltage and is entirely adequate to initiate an activation.) If the postsynaptic region is voltage-clamped, the value that reduces the membrane current to zero during transmitter release is called the reversal voltage Vr. One can show that it equals the average Nernst potential of sodium and potassium, as mentioned above. In the neuromuscular junction in skeletal muscle, this reversal voltage is about -15 mV.
0). What happens if the membrane becomes equally permeable to sodium and potassium ions? Such a condition would alter the membrane potential from near the potassium Nernst potential to a value that approximates the average of the sodium and potassium equilibrium potentials. (This potential, in turn, is close to zero transmembrane voltage and is entirely adequate to initiate an activation.) If the postsynaptic region is voltage-clamped, the value that reduces the membrane current to zero during transmitter release is called the reversal voltage Vr. One can show that it equals the average Nernst potential of sodium and potassium, as mentioned above. In the neuromuscular junction in skeletal muscle, this reversal voltage is about -15 mV.