6.1.1 Location of the Heart
The heart is located in the chest between the lungs behind the sternum and above the diaphragm. It is surrounded by the pericardium. Its size is about that of a fist, and its weight is about 250-300 g. Its center is located about 1.5 cm to the left of the midsagittal plane. Located above the heart are the great vessels: the superior and inferior vena cava, the pulmonary artery and vein, as well as the aorta. The aortic arch lies behind the heart. The esophagus and the spine lie further behind the heart. An overall view is given in Figure 6.1 (Williams and Warwick, 1989)..
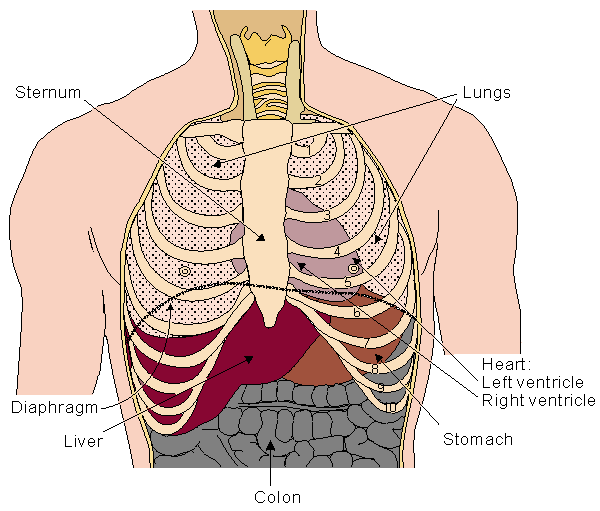
Fig. 6.1. Location of the heart in the thorax. It is bounded by the diaphragm, lungs, esophagus, descending aorta, and sternum.
6.1.2 Anatomy of the Heart
The walls of the heart are composed of cardiac muscle, called myocardium. It also has striations similar to skeletal muscle. It consists of four compartments: the right and left atria and ventricles. The heart is oriented so that the anterior aspect is the right ventricle while the posterior aspect shows the left atrium (see Figure 6.2). The atria form one unit and the ventricles another. This has special importance to the electric function of the heart, which will be discussed later. The left ventricular free wall and the septum are much thicker than the right ventricular wall. This is logical since the left ventricle pumps blood to the systemic circulation, where the pressure is considerably higher than for the pulmonary circulation, which arises from right ventricular outflow.
 The cardiac muscle fibers are oriented spirally (see Figure 6.3) and are divided into four groups: Two groups of fibers wind around the outside of both ventricles. Beneath these fibers a third group winds around both ventricles. Beneath these fibers a fourth group winds only around the left ventricle. The fact that cardiac muscle cells are oriented more tangentially than radially, and that the resistivity of the muscle is lower in the direction of the fiber has importance in electrocardiography and magnetocardiography.
The cardiac muscle fibers are oriented spirally (see Figure 6.3) and are divided into four groups: Two groups of fibers wind around the outside of both ventricles. Beneath these fibers a third group winds around both ventricles. Beneath these fibers a fourth group winds only around the left ventricle. The fact that cardiac muscle cells are oriented more tangentially than radially, and that the resistivity of the muscle is lower in the direction of the fiber has importance in electrocardiography and magnetocardiography.
 The heart has four valves. Between the right atrium and ventricle lies the tricuspid valve, and between the left atrium and ventricle is the mitral valve. The pulmonary valve lies between the right ventricle and the pulmonary artery, while the aortic valve lies in the outflow tract of the left ventricle (controlling flow to the aorta).
The heart has four valves. Between the right atrium and ventricle lies the tricuspid valve, and between the left atrium and ventricle is the mitral valve. The pulmonary valve lies between the right ventricle and the pulmonary artery, while the aortic valve lies in the outflow tract of the left ventricle (controlling flow to the aorta).
 The blood returns from the systemic circulation to the right atrium and from there goes through the tricuspid valve to the right ventricle. It is ejected from the right ventricle through the pulmonary valve to the lungs. Oxygenated blood returns from the lungs to the left atrium, and from there through the mitral valve to the left ventricle. Finally blood is pumped through the aortic valve to the aorta and the systemic circulation..
The blood returns from the systemic circulation to the right atrium and from there goes through the tricuspid valve to the right ventricle. It is ejected from the right ventricle through the pulmonary valve to the lungs. Oxygenated blood returns from the lungs to the left atrium, and from there through the mitral valve to the left ventricle. Finally blood is pumped through the aortic valve to the aorta and the systemic circulation..
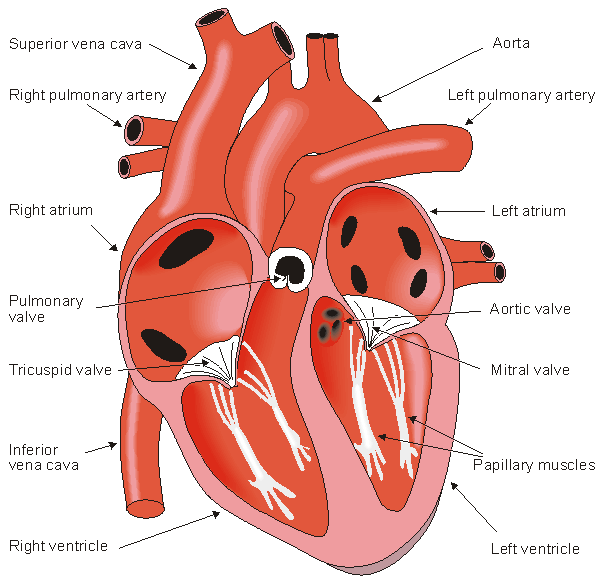
- Fig. 6.2. The anatomy of the heart and associated vessels.
6.2 ELECTRIC ACTIVATION OF THE HEART
6.2.1 Cardiac Muscle Cell
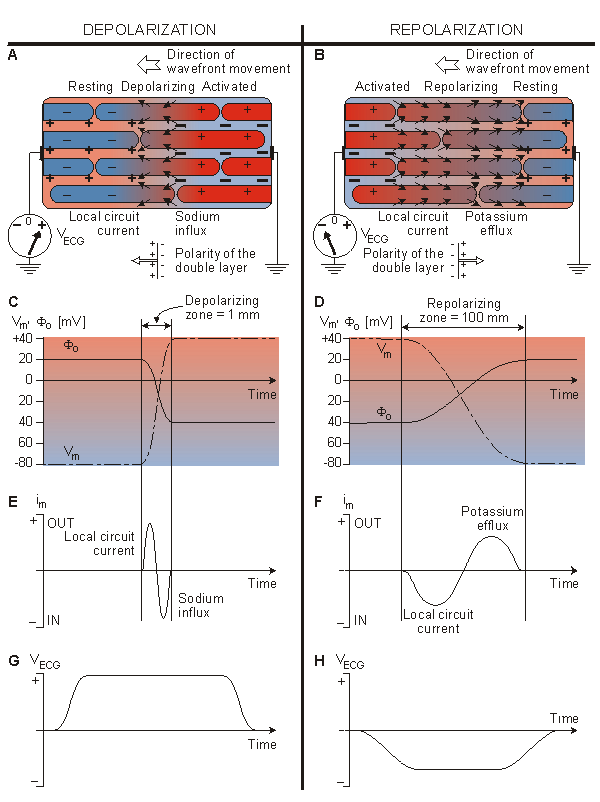
In the heart muscle cell, or myocyte, electric activation takes place by means of the same mechanism as in the nerve cell - that is, from the inflow of sodium ions across the cell membrane. The amplitude of the action potential is also similar, being about 100 mV for both nerve and muscle. The duration of the cardiac muscle impulse is, however, two orders of magnitude longer than that in either nerve cell or skeletal muscle. A plateau phase follows cardiac depolarization, and thereafter repolarization takes place. As in the nerve cell, repolarization is a consequence of the outflow of potassium ions. The duration of the action impulse is about 300 ms, as shown in Figure 6.4 (Netter, 1971).
6.2.2 The Conduction System of the Heart
Located in the right atrium at the superior vena cava is the sinus node (sinoatrial or SA node) which consists of specialized muscle cells. The sinoatrial node in humans is in the shape of a crescent and is about 15 mm long and 5 mm wide (see Figure 6.6). The SA nodal cells are self-excitatory, pacemaker cells. They generate an action potential at the rate of about 70 per minute. From the sinus node, activation propagates throughout the atria, but cannot propagate directly across the boundary between atria and ventricles, as noted above.
Because the intrinsic rate of the sinus node is the greatest, it sets the activation frequency of the whole heart. If the connection from the atria to the AV node fails, the AV node adopts its intrinsic frequency. If the conduction system fails at the bundle of His, the ventricles will beat at the rate determined by their own region that has the highest intrinsic frequency. The electric events in the heart are summarized in Table 6.1. The waveforms of action impulse observed in different specialized cardiac tissue are shown in Figure 6.7.
bundle of His
epicardium
epicardium
endocardium
depolarization
depolarization
repolarization
175
400
1.0-1.5
0.3 (axial)
Fig. 6.7. Electrophysiology of the heart.The different waveforms for each of the specialized cells found in the heart are shown. The latency shown approximates that normally found in the healthy heart.
A classical study of the propagation of excitation in human heart was made by Durrer and his co-workers (Durrer et al., 1970). They isolated the heart from a subject who had died of various cerebral conditions and who had no previous history of cardiac diseases. The heart was removed within 30 min post mortem and was perfused. As many as 870 electrodes were placed into the cardiac muscle; the electric activity was then recorded by a tape recorder and played back at a lower speed by the ECG writer; thus the effective paper speed was 960 mm/s, giving a time resolution better than 1 ms.
Figure 6.8 is redrawn from these experimental data. The ventricles are shown with the anterior wall of the left and partly that of the right ventricle opened. The isochronic surfaces show clearly that ventricular activation starts from the inner wall of the left ventricle and proceeds radially toward the epicardium. In the terminal part of ventricular activation, the excitation wavefront proceeds more tangentially. This phenomenon and its effects on electrocardiogram and magnetocardiogram signals are discussed in greater detail later.
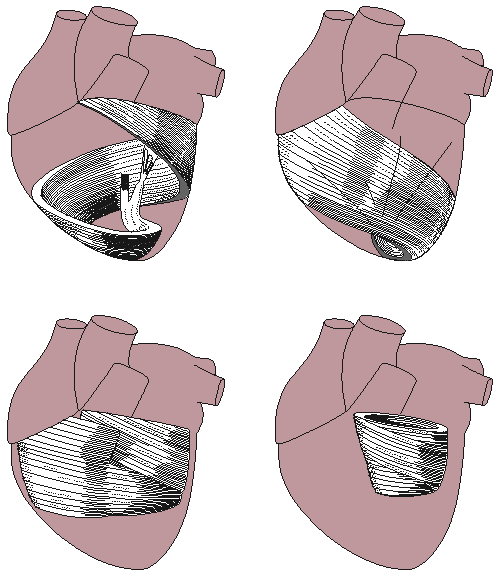
Fig. 6.3. Orientation of cardiac muscle fibers.
 Associated with the electric activation of cardiac muscle cell is its mechanical contraction, which occurs a little later. For the sake of comparison, Figure 6.5 illustrates the electric activity and mechanical contraction of frog sartorius muscle, frog cardiac muscle, and smooth muscle from the rat uterus (Ruch and Patton, 1982).
Associated with the electric activation of cardiac muscle cell is its mechanical contraction, which occurs a little later. For the sake of comparison, Figure 6.5 illustrates the electric activity and mechanical contraction of frog sartorius muscle, frog cardiac muscle, and smooth muscle from the rat uterus (Ruch and Patton, 1982).
 An important distinction between cardiac muscle tissue and skeletal muscle is that in cardiac muscle, activation can propagate from one cell to another in any direction. As a result, the activation wavefronts are of rather complex shape. The only exception is the boundary between the atria and ventricles, which the activation wave normally cannot cross except along a special conduction system, since a nonconducting barrier of fibrous tissue is present..
An important distinction between cardiac muscle tissue and skeletal muscle is that in cardiac muscle, activation can propagate from one cell to another in any direction. As a result, the activation wavefronts are of rather complex shape. The only exception is the boundary between the atria and ventricles, which the activation wave normally cannot cross except along a special conduction system, since a nonconducting barrier of fibrous tissue is present..
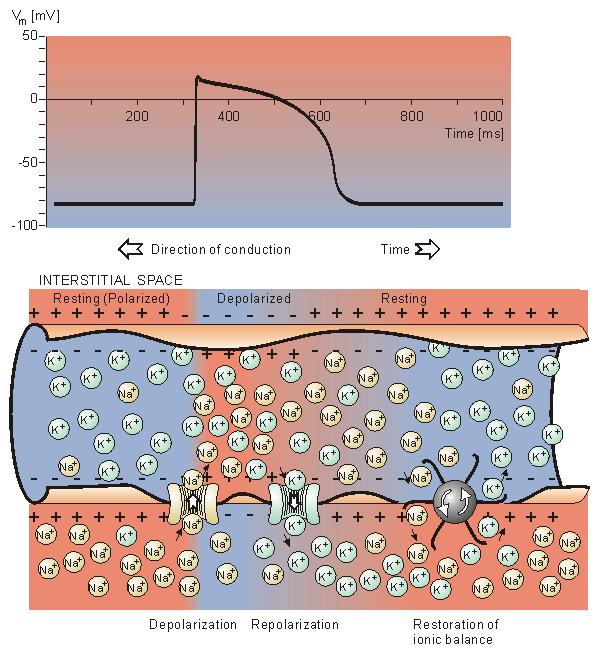
Fig. 6.4. Electrophysiology of the cardiac muscle cell.
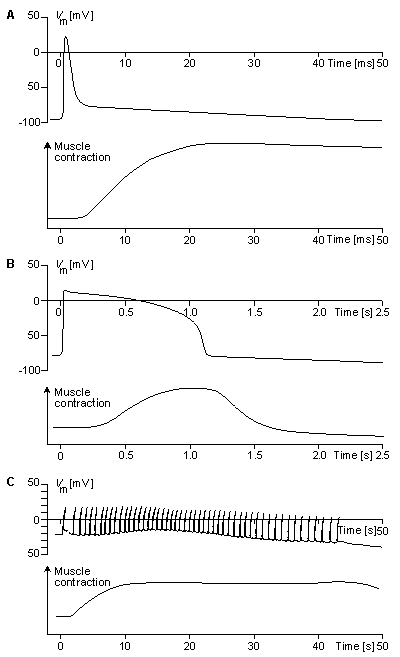
Fig. 6.5. Electric and mechanical activity in
 (A) frog sartorius muscle cell,
(A) frog sartorius muscle cell,
 (B) frog cardiac muscle cell, and
(B) frog cardiac muscle cell, and
 (C) rat uterus wall smooth muscle cell.
(C) rat uterus wall smooth muscle cell.
In each section the upper curve shows the transmembrane voltage behavior, whereas the lower one describes the mechanical contraction associated with it. The atrioventricular node (AV node) is located at the boundary between the atria and ventricles; it has an intrinsic frequency of about 50 pulses/min. However, if the AV node is triggered with a higher pulse frequency, it follows this higher frequency. In a normal heart, the AV node provides the only conducting path from the atria to the ventricles. Thus, under normal conditions, the latter can be excited only by pulses that propagate through it.
The atrioventricular node (AV node) is located at the boundary between the atria and ventricles; it has an intrinsic frequency of about 50 pulses/min. However, if the AV node is triggered with a higher pulse frequency, it follows this higher frequency. In a normal heart, the AV node provides the only conducting path from the atria to the ventricles. Thus, under normal conditions, the latter can be excited only by pulses that propagate through it.
 Propagation from the AV node to the ventricles is provided by a specialized conduction system. Proximally, this system is composed of a common bundle, called the bundle of His (named after German physician Wilhelm His, Jr., 1863-1934). More distally, it separates into two bundle branches propagating along each side of the septum, constituting the right and left bundle branches. (The left bundle subsequently divides into an anterior and posterior branch.) Even more distally the bundles ramify into Purkinje fibers (named after Jan Evangelista Purkinje (Czech; 1787-1869)) that diverge to the inner sides of the ventricular walls. Propagation along the conduction system takes place at a relatively high speed once it is within the ventricular region, but prior to this (through the AV node) the velocity is extremely slow.
Propagation from the AV node to the ventricles is provided by a specialized conduction system. Proximally, this system is composed of a common bundle, called the bundle of His (named after German physician Wilhelm His, Jr., 1863-1934). More distally, it separates into two bundle branches propagating along each side of the septum, constituting the right and left bundle branches. (The left bundle subsequently divides into an anterior and posterior branch.) Even more distally the bundles ramify into Purkinje fibers (named after Jan Evangelista Purkinje (Czech; 1787-1869)) that diverge to the inner sides of the ventricular walls. Propagation along the conduction system takes place at a relatively high speed once it is within the ventricular region, but prior to this (through the AV node) the velocity is extremely slow.
 From the inner side of the ventricular wall, the many activation sites cause the formation of a wavefront which propagates through the ventricular mass toward the outer wall. This process results from cell-to-cell activation. After each ventricular muscle region has depolarized, repolarization occurs. Repolarization is not a propagating phenomenon, and because the duration of the action impulse is much shorter at the epicardium (the outer side of the cardiac muscle) than at the endocardium (the inner side of the cardiac muscle), the termination of activity appears as if it were propagating from epicardium toward the endocardium.
From the inner side of the ventricular wall, the many activation sites cause the formation of a wavefront which propagates through the ventricular mass toward the outer wall. This process results from cell-to-cell activation. After each ventricular muscle region has depolarized, repolarization occurs. Repolarization is not a propagating phenomenon, and because the duration of the action impulse is much shorter at the epicardium (the outer side of the cardiac muscle) than at the endocardium (the inner side of the cardiac muscle), the termination of activity appears as if it were propagating from epicardium toward the endocardium.
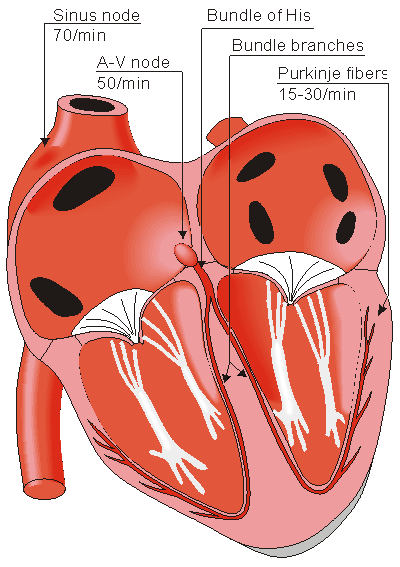
Fig. 6.6. The conduction system of the heart.
Location in
the heartEvent Time [ms]
ECG-
terminologyConduction
velocity [m/s] Intrinsic
frequency [1/min]
SA node
atrium, Right
Left
AV node
bundle branches
Purkinje fibers
endocardium
Septum
Left ventricle
Left ventricle
Right ventricle
Left ventricle
Right ventricle
Left ventricle
impulse generated
depolarization *)
depolarization
arrival of impulse
departure of impulse
activated
activated
activated
depolarization
depolarization
repolarization
repolarization
0
5
85
50
125
130
145
150
190
225
250
600



P
P
P-Q
interval
QRS
T
0.05
0.8-1.0
0.8-1.0
0.02-0.05
1.0-1.5
3.0-3.5
-
0.8
(transverse)
0.5
70-80
20-40
*) Atrial repolarization occurs during the ventricular depolarization; therefore, it is not normally seen in the electrocardiogram.
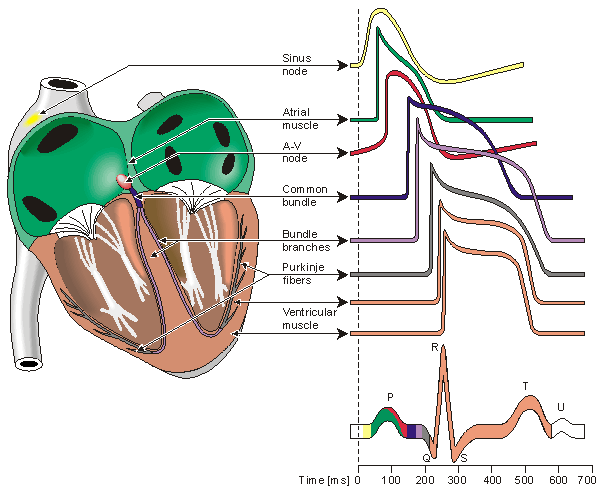
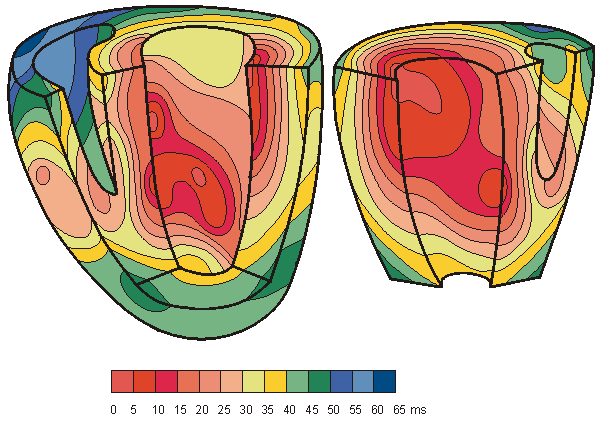
Fig. 6.8. Isochronic surfaces of the ventricular activation. (From Durrer et al., 1970.)






 , to x = x gives
, to x = x gives