Many investigations in electrophysiology involve preparations that contain multiple cells. Examples include the nerve bundle, which consists of several thousand myelinated fibers; striated whole muscle, which may contain several thousand individual fibers; the heart, which has on the order of 1010 cells; and the brain, which also has about 1010 cells. In modeling the electric behavior of such preparations, the discrete cellular structure may be important (Spach, 1983). On the other hand, macroscopic (averaged) fields may adequately describe the phenomena of interest. In the latter case it is possible to replace the discrete structure with an averaged continuum that represents a considerable simplification. The goal of this chapter is to formulate a continuum representation of multicellular systems and then to explore its electric properties.
10.1.1 Electronic Modeling of Excitable Tissue
In Chapters 3 and 4, we discussed the electric behavior of excitable tissues - the nerve and the muscle cell. In that discussion we have used equations that describe the equivalent electric circuit of the membrane as well as electronic circuits that represent the passive electric properties of the tissue. From these equations and electric circuits we utilize the following:
The Nernst equation (Equation 3.21), which expresses the required membrane voltage to equilibrate the ion flux through the membrane for an existing concentration ratio of a particular ion species. Because the Nernst equation evaluates the ion moving force due to a concentration gradient as a voltage [V], this may be represented in equivalent electric circuits as a battery.
The cable model of an axon, which is composed of external and internal resistances as well as the electric properties of the membrane. This equivalent circuit may be used to calculate the general cable equation of the axon (Equation 3.45) describing the subthreshold transmembrane voltage response to a constant current stimulation. The time-varying equations describing the behavior of the transmembrane voltage due to a step-impulse stimulation are also of interest (though more complicated). Their solutions were illustrated in Figure 3.11. The equivalent circuit for (approximate) derivation of the strength-duration equation, Equation 3.58, was shown in Figure 3.12.
The equivalent electric circuits describing the behavior of the axon under conditions of nerve propagation, or under space-clamp and voltage-clamp conditions, are shown in Figures 4.1, 4.2, and 4.3; the corresponding equations are 4.1, 4.2, and 4.3, respectively.
The electric circuit for the parallel-conductance model of the membrane, which contains pathways for sodium, potassium, and chloride ion currents, is illustrated in Figure 4.10, and its behavior described by Equation 4.10. This equation includes the following passive electric parameters (electronic components): membrane capacitance, Nernst voltages for sodium, potassium, and chloride ions, as well as the leakage conductance. Further, the circuit includes the behavior of the active parameters, the sodium and potassium conductances, as described by the Hodgkin-Huxley equations (Equations 4.12-4.24).
 Thus our understanding of the electric behavior of excitable tissue, and our methods to illustrate it are strongly tied to the concepts of electronic circuits and to the equations describing their behavior. From this standpoint it is possible to proceed to realize physically the electronic equivalent circuits for the excitable tissues. The physical realization of the electronic equivalent circuits of excitable tissues has two main purposes:
Thus our understanding of the electric behavior of excitable tissue, and our methods to illustrate it are strongly tied to the concepts of electronic circuits and to the equations describing their behavior. From this standpoint it is possible to proceed to realize physically the electronic equivalent circuits for the excitable tissues. The physical realization of the electronic equivalent circuits of excitable tissues has two main purposes:
It provides us with an opportunity to verify that the models we have constructed really behave the same as the excitable tissue that they should model - that is, that the model is correct. If the behavior of the model is not completely correct, we may be able to adjust the properties of the model and thereby improve our understanding of the behavior of the tissue. This analysis of the behavior of the excitable tissue is one general purpose of modeling work.
There exists also the possibility of constructing, or synthesizing electronic circuits, whose behavior is similar to neural tissue, and which perform information processing in a way that also is similar to nature. In its most advanced form, it is called neural computing.
 In Section 7.3 we discussed the concept of modeling in general. Various models in the neurosciences are discussed in Miller (1992). In this chapter, we discuss especially electronic neural modeling including representative examples of electronic neuron models developed to realize the electric behavior of neurons. A more comprehensive review of the electronic neuron models constructed with discrete electronic components may be found in Malmivuo (1973) and Reiss et al. (1964).
In Section 7.3 we discussed the concept of modeling in general. Various models in the neurosciences are discussed in Miller (1992). In this chapter, we discuss especially electronic neural modeling including representative examples of electronic neuron models developed to realize the electric behavior of neurons. A more comprehensive review of the electronic neuron models constructed with discrete electronic components may be found in Malmivuo (1973) and Reiss et al. (1964).
 We should note that simulation of electric circuits with digital computers is another way to investigate the behavior of the electronic models. Despite this fact, electronic neural modeling is important because it is the bridge to construction of electronic circuits, which are the elements of neurocomputers.
We should note that simulation of electric circuits with digital computers is another way to investigate the behavior of the electronic models. Despite this fact, electronic neural modeling is important because it is the bridge to construction of electronic circuits, which are the elements of neurocomputers.
10.1.2 Neurocomputers
The most important application of electronic neural modeling is the neurocomputer. Although this subject is beyond the scope of this book, and the theory of neural networks and neurocomputers is not discussed in this volume, we include here a brief description and include some references to this subject. A good introduction and short review is in Hecht-Nielsen (1988).
 The first computers were called "electric brains." At that time, there was a popular conception that computers could think, or that such computers would soon be available. In reality, however, even computers of today must be programmed exactly to do the desired task.
The first computers were called "electric brains." At that time, there was a popular conception that computers could think, or that such computers would soon be available. In reality, however, even computers of today must be programmed exactly to do the desired task.
 Artificial intelligence has been a popular buzzword for decades. It has produced some useful expert systems, chess-playing programs, and some limited speech and character recognition systems. These remain in the domain of carefully crafted algorithmic programs that perform a specific task. A self-programming computer does not exist. The Turing test of machine intelligence is that a machine is intelligent if in conversing with it, one is unable to tell whether one is talking to a human or a machine. By this criterion artificial intelligence does not seem any closer to realization than it was 30 years ago.
Artificial intelligence has been a popular buzzword for decades. It has produced some useful expert systems, chess-playing programs, and some limited speech and character recognition systems. These remain in the domain of carefully crafted algorithmic programs that perform a specific task. A self-programming computer does not exist. The Turing test of machine intelligence is that a machine is intelligent if in conversing with it, one is unable to tell whether one is talking to a human or a machine. By this criterion artificial intelligence does not seem any closer to realization than it was 30 years ago.
 If we make an attempt to build an electronic brain, it makes sense to study how a biological brain works and then to try to imitate nature. This idea has not been ignored by scientists. Real brains, even those of primitive animals, are, however, enormously complex structures. The human brain contains about 1011 neurons, each capable of storing more than a single bit of data.
If we make an attempt to build an electronic brain, it makes sense to study how a biological brain works and then to try to imitate nature. This idea has not been ignored by scientists. Real brains, even those of primitive animals, are, however, enormously complex structures. The human brain contains about 1011 neurons, each capable of storing more than a single bit of data.
 Computers are approaching the point at which they could have a comparable memory capacity. Whereas computer instruction times are measured in nanoseconds, mammalian information processing is done in milliseconds. However, this speed advantage for the computer is superseded by the massively parallel structure of the nervous system; each neuron processes information and has a large number of interconnections to other neurons. Multiprocessor computers are now being built, but making effective use of thousands of processors is a task that is still a challenge for computer theory (Tonk and Hopfield, 1987).
Computers are approaching the point at which they could have a comparable memory capacity. Whereas computer instruction times are measured in nanoseconds, mammalian information processing is done in milliseconds. However, this speed advantage for the computer is superseded by the massively parallel structure of the nervous system; each neuron processes information and has a large number of interconnections to other neurons. Multiprocessor computers are now being built, but making effective use of thousands of processors is a task that is still a challenge for computer theory (Tonk and Hopfield, 1987).
10.2 CLASSIFICATION OF NEURON MODELS
In general, neuron models may be divided into categories according to many different criteria. In the following, four different criteria are presented, to exemplify these classifications (Malmivuo, 1973):
10.3 MODELS DESCRIBING THE FUNCTION OF THE MEMBRANE
Most of the models describing the excitation mechanism of the membrane are electronic realizations of the theoretical membrane model of Hodgkin and Huxley (Hodgkin and Huxley, 1952). In the following sections, two of these realizations are discussed.
10.3.1 The Lewis Membrane Model
Edwin R. Lewis published several electronic membrane models that are based on the Hodgkin-Huxley equations. The sodium and potassium conductances, synaptic connections, and other functions of the model are realized with discrete transistors and associated components. All these are parallel circuits connected between nodes representing the inside and outside of the membrane.
Potassium Conductance
Sodium Conductance
Simulated Action Pulse
10.3.2 The Roy Membrane Model
Guy Roy published an electronic membrane model in 1972 (Roy, 1972) and gave it the name "Neurofet." His model, analogous to Lewis's, is also based on the Hodgkin-Huxley model. Roy used FET transistors to simulate the sodium and potassium conductances. FETs are well known as adjustable conductors. So the multiplying circuit of Lewis may be incorporated into a single FET component (Figure 10.6).
10.4 MODELS DESCRIBING THE CELL AS AN INDEPENDENT UNIT
10.4.1 The Lewis Neuron Model
In this section the neuron model described by Lewis in 1968 (Lewis, 1968) is briefly discussed. The Lewis model is based on the Hodgkin-Huxley membrane model and the theories of Eccles on synaptic transmission (Eccles, 1964). The model circuit is illustrated in Figure 10.9.
Figure 10.11A and 10.11B compare the potassium and sodium ion currents of the Lewis model to those in the Hodgkin-Huxley model, respectively. Figure 10.12 illustrates the action pulse generated by the Lewis model. The peak magnitude of the simulated sodium current is 10 mA. This magnitude is equivalent to approximately 450 µA/cm2 in the membrane, which is about half of the value calculated by Hodgkin and Huxley from their model. The maximum potassium current in the circuit is 3 mA, corresponding to 135 µA/cm2 in the membrane. The author gave no calibration for the membrane potential or for the time axis.
The electronic realizations of the Hodgkin-Huxley model are very accurate in simulating the function of a single neuron. However, when one is trying to simulate the function of neural networks, they become very complicated. Many scientists feel that when simulating large neural networks, the internal construction of its element may not be too important. It may be satisfactory simply to ensure that the elements produce an action pulse in response to the stimuli in a manner similar to an actual neuron. On this basis, Leon D. Harmon constructed a neuron model having a very simple circuit. With this model he performed experiments in which he simulated many functions characteristic of the neuron (Harmon, 1961).
Strength-Duration Curve
Latency
Temporal Summation
Refractory Period (Recovery of Excitability)
Output Pulse, Action Impulse
Delay
Repetitive Excitation
10.5 A MODEL DESCRIBING THE PROPAGATION OF ACTION PULSE IN AXON
Using an iteration of the membrane section of his neuron model described in Section 10.3.1, Lewis simulated the propagation of an action pulse in a uniform axon and obtained interesting results (Lewis, 1968). The model structure, illustrated in Figure 10.15, can be seen to include a network of membrane elements as well as axial resistors representing the intracellular resistance. A total of six membrane elements are depicted in the figure. The model is an electronic realization of the linear core-conductor model with active membrane elements.
10.6 INTEGRATED CIRCUIT REALIZATIONS
The development of the integrated circuit technology has made it possible to produce electronic neuron models in large quantities (Mahowald et al., 1992). This makes it possible to use electronic neuron models or neuron-like circuits as processing elements for electronic computers, called neurocomputers. In the following paragraphs, we give two examples of these.
(1) The membrane in the resting state
(2) The mechanism generating the nerve impulse
(3) The propagation of the nerve impulse in an axon
 This chapter considers representative examples of electronic neuron models (or neuromimes) that describe the generation of the action impulse, the neuron as an independent unit, and the propagation of the nerve impulse in the axon.
This chapter considers representative examples of electronic neuron models (or neuromimes) that describe the generation of the action impulse, the neuron as an independent unit, and the propagation of the nerve impulse in the axon.
 We discuss here the model published by Lewis in 1964. Lewis realized the sodium and potassium conductances using electronic hardware in the form of active filters, as shown in the block diagram of Figure 10.1. Since the output of the model is the transmembrane voltage Vm, the potassium current can be evaluated by multiplying the voltage corresponding to GK by (Vm - VK). Figure 10.1 is consequently an accurate physical analog to the Hodgkin-Huxley expressions, and the behavior of the output voltage Vm corresponds to that predicted by the Hodgkin-Huxley equations.
We discuss here the model published by Lewis in 1964. Lewis realized the sodium and potassium conductances using electronic hardware in the form of active filters, as shown in the block diagram of Figure 10.1. Since the output of the model is the transmembrane voltage Vm, the potassium current can be evaluated by multiplying the voltage corresponding to GK by (Vm - VK). Figure 10.1 is consequently an accurate physical analog to the Hodgkin-Huxley expressions, and the behavior of the output voltage Vm corresponds to that predicted by the Hodgkin-Huxley equations.
 The electronic circuits in the Lewis neuromime had provision for inserting (and varying) not only such constants as GK max, GNa max, VK, VNa, VCl, which enter the Hodgkin-Huxley formulation, but also th, tm, tn, which allow modifications from the Hodgkin-Huxley equations. The goal of Lewis's research was to simulate the behavior of a neuronal network, including coupled neurons, each of which is simulated by a neuromime; this is documented later in this chapter.
The electronic circuits in the Lewis neuromime had provision for inserting (and varying) not only such constants as GK max, GNa max, VK, VNa, VCl, which enter the Hodgkin-Huxley formulation, but also th, tm, tn, which allow modifications from the Hodgkin-Huxley equations. The goal of Lewis's research was to simulate the behavior of a neuronal network, including coupled neurons, each of which is simulated by a neuromime; this is documented later in this chapter.
 In the electronic realization the voltages of the biological membrane are multiplied by 100 to fit the electronic circuit. In other quantities, the original values of the biological membrane have been used. In the following, the components of the model are discussed separately.
In the electronic realization the voltages of the biological membrane are multiplied by 100 to fit the electronic circuit. In other quantities, the original values of the biological membrane have been used. In the following, the components of the model are discussed separately.
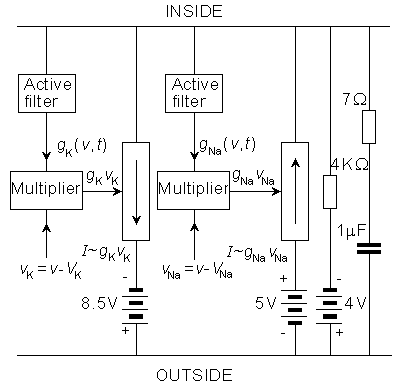
Fig. 10.1. The block diagram of the Lewis membrane model.
The circuit simulating the potassium conductance is shown in Figure 10.2. The potassium conductance function GK(Vm,t) is generated from the simulated membrane voltage through a nonlinear active filter according to the Hodgkin-Huxley model (in the figure separated with a dashed line). The three variable resistors in the filter provide a control over the delay time, rise time, and fall time. The value of the potassium conductance is adjusted with a potentiometer, which is the amplitude regulator of a multiplier. The multiplier circuit generates the function GK(Vm,t)·vK, where vK is the difference between the potassium potential (VK) and membrane potential (Vm). The multiplier is based on the quadratic function of two diodes.
In the circuit simulating the sodium conductance, Lewis omitted the multiplier on the basis that the equilibrium voltage of sodium ions is about 120 mV more positive than the resting voltage. Because we are more interested in small membrane voltage changes, the gradient of sodium ions may be considered constant. The circuit simulating the sodium conductance is shown in Figure 10.3. The time constant of the inactivation is defined according to a varistor. The inactivation decreases monotonically with the depolarization, approximately following the Hodgkin-Huxley model.
By connecting the components of the membrane model as in Figure 10.4 and stimulating the model analogously to the real axon, the model generates a membrane action pulse. This simulated action pulse follows the natural action pulse very accurately. Figure 10.5A illustrates a single action pulse generated by the Lewis membrane model, and Figure 10.5B shows a series of action pulses..
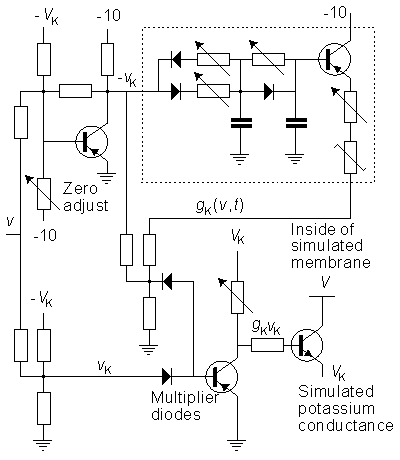
Fig. 10.2. The circuit simulating the potassium conductance of the Lewis membrane model.
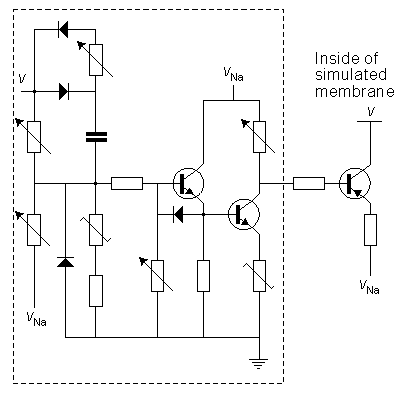
Fig. 10.3. Circuit simulating the sodium conductance of the Lewis membrane model.
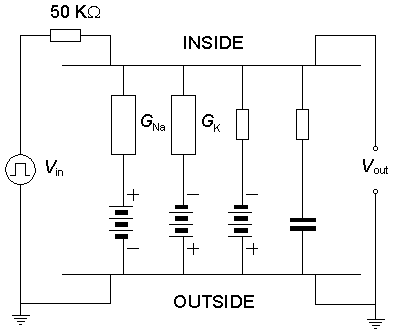
Fig. 10.4. The complete Lewis membrane model.
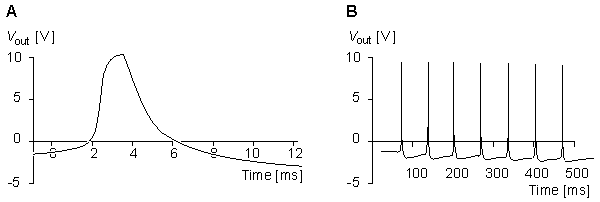
Fig. 10.5. (A) Single action pulse, and (B) a series of action pulses generated by the Lewis membrane model.
 In the Roy model the conductance is controlled by a circuit including an operational amplifier, capacitors, and resistors. This circuit is designed to make the conductance behave according to the Hodgkin-Huxley model. Roy's main goal was to achieve a very simple model rather than to simulate accurately the Hodgkin-Huxley model. Nevertheless, the measurements resulting from his model, shown in Figures 10.7 and 10.8, are reasonably close to the results obtained by Hodgkin and Huxley.
In the Roy model the conductance is controlled by a circuit including an operational amplifier, capacitors, and resistors. This circuit is designed to make the conductance behave according to the Hodgkin-Huxley model. Roy's main goal was to achieve a very simple model rather than to simulate accurately the Hodgkin-Huxley model. Nevertheless, the measurements resulting from his model, shown in Figures 10.7 and 10.8, are reasonably close to the results obtained by Hodgkin and Huxley.
 Figure 10.7 illustrates the steady-state values for the potassium and sodium conductances as a function of applied voltage. Note that for potassium conductance the value given is the steady-state value, which it reaches in steady state. For sodium the illustrated value is
Figure 10.7 illustrates the steady-state values for the potassium and sodium conductances as a function of applied voltage. Note that for potassium conductance the value given is the steady-state value, which it reaches in steady state. For sodium the illustrated value is 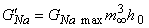 ; it is the value that the sodium conductance would attain if h remained at its resting level (h0). (The potassium and sodium conductance values of Hodgkin and Huxley are from tables 1 and 2, respectively, in Hodgkin and Huxley, 1952.)
; it is the value that the sodium conductance would attain if h remained at its resting level (h0). (The potassium and sodium conductance values of Hodgkin and Huxley are from tables 1 and 2, respectively, in Hodgkin and Huxley, 1952.)
 The full membrane model was obtained by connecting the potassium and sodium conductances in series with their respective batteries and simulating the membrane capacitance with a capacitor of 4.7 nF and simulating the leakage conductance with a resistance of 200 Wk . The results from the simulation of the action pulse are illustrated in Figure 10.8..
The full membrane model was obtained by connecting the potassium and sodium conductances in series with their respective batteries and simulating the membrane capacitance with a capacitor of 4.7 nF and simulating the leakage conductance with a resistance of 200 Wk . The results from the simulation of the action pulse are illustrated in Figure 10.8..
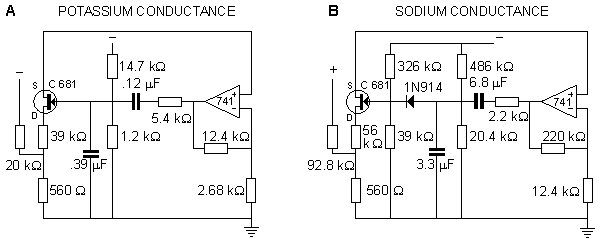
Fig. 10.6. The circuits simulating (A) sodium and (B) potassium conductances in the Roy membrane model.
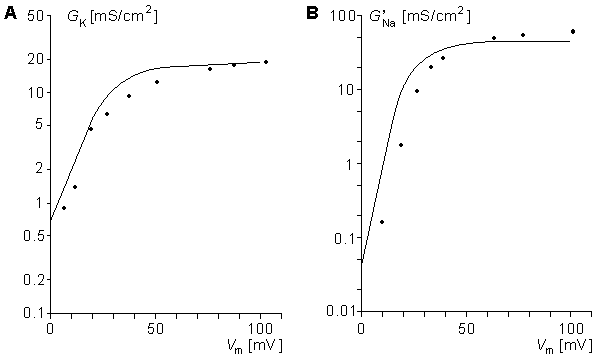
Fig. 10.7. Steady-state values of the (A) GK and (B) G'Na as a function of membrane voltage clamp in the Roy model (solid lines), compared to the measurements of Hodgkin and Huxley (dots). Vm, the transmembrane voltage, is related to the resting value of the applied voltage clamp. (See the text for details.)

Fig. 10.8. Voltage-clamp measurements made for (A) potassium and (B) sodium conductances in the Roy model. The voltage steps are 20, 40, 60, 80, and 100 mV. (C) The action pulse simulated with the Roy model.
 This neuron model is divided into two sections: the synaptic section and the section generating the action pulse. Both sections consist of parallel circuits connected to the nodes representing the intracellular and extracellular sites of the membrane.
This neuron model is divided into two sections: the synaptic section and the section generating the action pulse. Both sections consist of parallel circuits connected to the nodes representing the intracellular and extracellular sites of the membrane.
 The section representing the synaptic junction is divided into two components. One of these represents the inhibitory junction and the other the excitatory junction. The sensitivity of the section generating the action pulse to a stimulus introduced at the excitatory synaptic junction is reduced by the voltage introduced at the inhibitory junction. The section generating the action pulse is based on the Hodgkin-Huxley model. As described earlier, it consists of the normal circuits simulating the sodium and potassium conductances, the leakage conductance, and the membrane capacitance. The circuit also includes an amplifier for the output signal.
The section representing the synaptic junction is divided into two components. One of these represents the inhibitory junction and the other the excitatory junction. The sensitivity of the section generating the action pulse to a stimulus introduced at the excitatory synaptic junction is reduced by the voltage introduced at the inhibitory junction. The section generating the action pulse is based on the Hodgkin-Huxley model. As described earlier, it consists of the normal circuits simulating the sodium and potassium conductances, the leakage conductance, and the membrane capacitance. The circuit also includes an amplifier for the output signal.
 This neuron model which is relatively complicated, is to be used in research on neural networks. However, it is actually a simplified version of Lewis's 46-transistor network having the same form. The purpose of this simplified Lewis model is to simulate the form of the action pulse, not with the highest possible accuracy but, rather, with a sufficient accuracy provided by a simple model. Figures 10.10, 10.11, and 10.12 show the behavior of the model compared to the simulation based directly on the Hodgkin and Huxley model.
This neuron model which is relatively complicated, is to be used in research on neural networks. However, it is actually a simplified version of Lewis's 46-transistor network having the same form. The purpose of this simplified Lewis model is to simulate the form of the action pulse, not with the highest possible accuracy but, rather, with a sufficient accuracy provided by a simple model. Figures 10.10, 10.11, and 10.12 show the behavior of the model compared to the simulation based directly on the Hodgkin and Huxley model.
 From Figure 10.10 we find that when the stimulation current begins, the sodium ion current determined by Lewis (I'Na) rises to its peak value almost immediately, whereas the sodium ion current of the Hodgkin-Huxley biological nerve (INa) rises much more slowly. The exponential decay of the current occurs at about the same speed in both. The behavior of the potassium ion current is very similar in both the model and the biological membrane as simulated by Hodgkin and Huxley.
From Figure 10.10 we find that when the stimulation current begins, the sodium ion current determined by Lewis (I'Na) rises to its peak value almost immediately, whereas the sodium ion current of the Hodgkin-Huxley biological nerve (INa) rises much more slowly. The exponential decay of the current occurs at about the same speed in both. The behavior of the potassium ion current is very similar in both the model and the biological membrane as simulated by Hodgkin and Huxley.
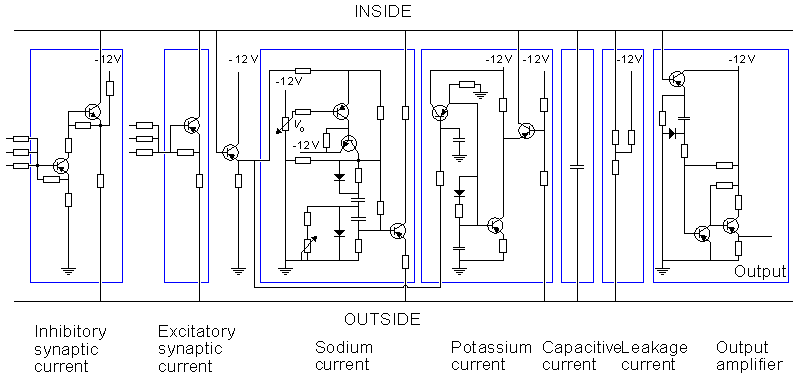
Fig. 10.9. The Lewis neuron model from 1968.
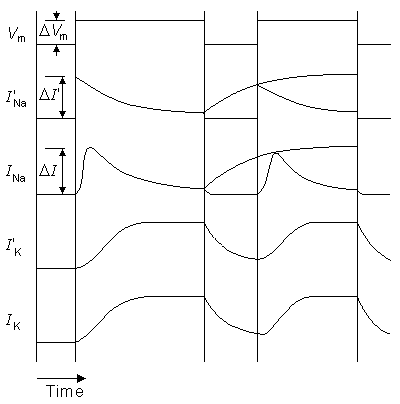
Fig. 10.10. The responses of the sodium and potassium current from the Lewis model (primed) and the biological neuron (as evaluated by the Hodgkin-Huxley model) to a voltage step. The applied transmembrane voltage is shown as Vm
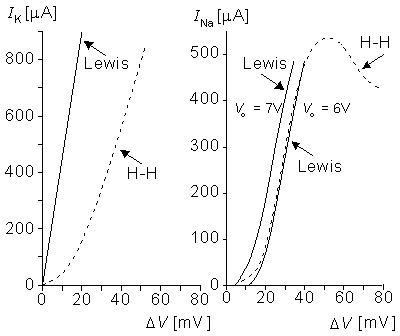
Fig. 10.11. (A) Steady-state potassium and (B) peak sodium currents in response to Vm determined in the Lewis model (solid line) and in the simulation based directly on the Hodgkin and Huxley model (dashed line) as a function of the membrane voltage. (Vo is the voltage applied by the potentiometer in the sodium current circuit.)
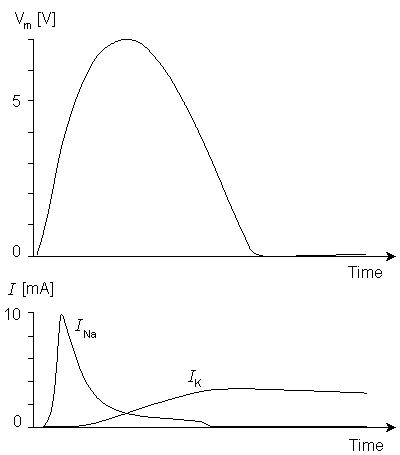
Fig. 10.12. The action pulse generated by the Lewis model. The corresponding sodium and potassium currents are also illustrated.
10.4.2 The Harmon Neuron Model
 The circuit of the Harmon neuron model is given in Figure 10.13. Figures 10.13A and 10.13B show the preliminary and more advanced versions of the circuit, respectively. The model is equipped with five excitatory inputs which can be adjusted. These include diode circuits representing various synaptic functions. The signal introduced at excitatory inputs charges the 0.02 µF capacitor which, after reaching a voltage of about 1.5 V, allows the monostable multivibrator, formed by transistors T1 and T2, to generate the action pulse. This impulse is amplified by transistors T3 and T4. The output of one neuron model may drive the inputs of perhaps 100 neighboring neuron models. The basic model also includes an inhibitory input. A pulse introduced at this input has the effect of decreasing the sensitivity to the excitatory inputs..
The circuit of the Harmon neuron model is given in Figure 10.13. Figures 10.13A and 10.13B show the preliminary and more advanced versions of the circuit, respectively. The model is equipped with five excitatory inputs which can be adjusted. These include diode circuits representing various synaptic functions. The signal introduced at excitatory inputs charges the 0.02 µF capacitor which, after reaching a voltage of about 1.5 V, allows the monostable multivibrator, formed by transistors T1 and T2, to generate the action pulse. This impulse is amplified by transistors T3 and T4. The output of one neuron model may drive the inputs of perhaps 100 neighboring neuron models. The basic model also includes an inhibitory input. A pulse introduced at this input has the effect of decreasing the sensitivity to the excitatory inputs..
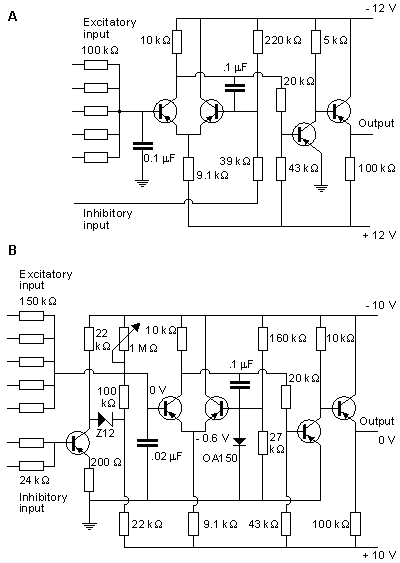
Fig. 10.13. Construction of the Harmon neuron model. (A) The preliminary and (B) the more advanced version of the circuit.. Without external circuits, Harmon investigated successfully seven properties of his neuron model. These are illustrated in Figure 10.14 and are described briefly in the following.
The Harmon model follows a strength-duration curve similar to that exhibited by the natural neuron. The time scale is approximately correct, but owing to the electric properties of circuit components, the voltage scale is much higher. The threshold voltage in the Harmon model is about Vth = 1.5 V, as described in Figure 10.14A.
Because the model contains no internal circuit that specifically generates a latency, this phenomenon is totally described by the strength-duration curve which is interpreted as a stimulus-latency curve. The action pulse is generated only when the stimulus has lasted long enough to generate the action pulse.
The model illustrates the stimulus threshold in the case of two consecutive stimulus pulses where the first pulse leaves the membrane hyperexcitable to the second. Figure 10.14B shows the required amplitude of two 0.8 ms pulses as a function of their interval, and one notes that the threshold diminishes with a reduced pulse interval, owing to temporal summation. In all cases the pulse amplitude is reduced from the value required for activation from a single pulse.
The typical recovery of excitability of the model after an action pulse is shown in Figure 10.14C (curve A). This curve is similar to that for a biological neuron. The neuron model is absolutely refractory for about 1 ms - that is, the time of the output pulse. The relative refractory period starts after this (t = 0), and its time constant is about 1.7 ms. Curve A is obtained when the stimulus is applied at one input. Curve B represents the situation when the stimulus is simultaneously applied to three inputs (see Fig. 10.13).
The output pulse obeys the all-or-none law, and its amplitude is quite stable. Its width is, however, to some degree a function of the pulse frequency. This dependence is given in Figure 10.14D.
The delay refers, in this case, to the time between the onsets of the stimulus pulse and the output pulse. It is not the delay in the usual meaning of the term. In the model, the delay is a function of the integration in the input as well as the refractory condition. Curve A in Figure 10.14E represents the delay when a stimulus is applied to one input, and curve B when the stimulus is applied to all five excitatory inputs.
Repetitive excitation refers to the generation of output pulses with a constant input voltage and frequency. Figure 10.14F, curve A, shows the frequency of the output pulse when the input voltage is connected to three inputs, and curve B when the input voltage is connected to one input. The output frequency follows the input only for high voltage inputs. As the input is reduced, pulses drop out, and the resulting output frequency is reduced compared to the input.
 By connecting capacitors between the input and output ports of the neuron model, it is possible to realize much more complex functions. Harmon performed experiments also with combinations of many neuron models. Furthermore, Harmon investigated propagation of the action pulse by chaining models together. These neuron models can be applied to simulate quite complex neural networks, and even to model brain waves.
By connecting capacitors between the input and output ports of the neuron model, it is possible to realize much more complex functions. Harmon performed experiments also with combinations of many neuron models. Furthermore, Harmon investigated propagation of the action pulse by chaining models together. These neuron models can be applied to simulate quite complex neural networks, and even to model brain waves.
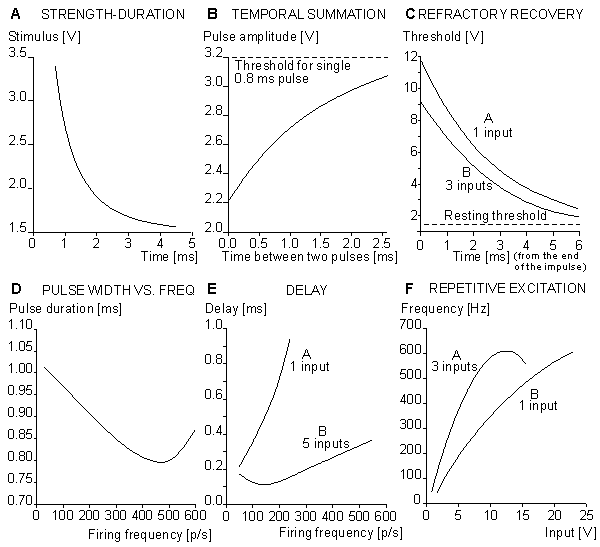
Fig. 10.14. Properties of the Harmon model.
 (A) strength-duration curve;
(A) strength-duration curve;
 (B) temporal summation of the stimulus;
(B) temporal summation of the stimulus;
 (C) refractory recovery;
(C) refractory recovery;
 (D) the output pulse width as a function of the pulse frequency;
(D) the output pulse width as a function of the pulse frequency;
 (E) the delay between initiation of excitation and initiation of the action pulse as a function of firing frequency; and
(E) the delay between initiation of excitation and initiation of the action pulse as a function of firing frequency; and
 (F) the behavior of the model in repetitive excitation. The input frequency is 700 p/s.
(F) the behavior of the model in repetitive excitation. The input frequency is 700 p/s. Figures 10.16A and 10.16B illustrate the simulation of propagation of an action pulse in a model consisting of a chain of axon units, as described in Figure 10.15. Curve A represents the case with a chain of six units, and curve B a continuous ring of 10 units. In the latter, the signals are recorded from every second unit. A six-unit model represents a section of a squid axon 4 cm long and 1 mm in diameter. Figure 10.15A shows that the conduction time of the action pulse from unit three (where it has reached the final form) to unit six (i.e., three increments of distance) is 1.4 ms. Because the full six-unit model forms five increments of distance, the modeled conduction velocity was 17 m/s. This is comparable to spike conduction velocities (14 - 23 m/s) measured in squid giant axons..
Figures 10.16A and 10.16B illustrate the simulation of propagation of an action pulse in a model consisting of a chain of axon units, as described in Figure 10.15. Curve A represents the case with a chain of six units, and curve B a continuous ring of 10 units. In the latter, the signals are recorded from every second unit. A six-unit model represents a section of a squid axon 4 cm long and 1 mm in diameter. Figure 10.15A shows that the conduction time of the action pulse from unit three (where it has reached the final form) to unit six (i.e., three increments of distance) is 1.4 ms. Because the full six-unit model forms five increments of distance, the modeled conduction velocity was 17 m/s. This is comparable to spike conduction velocities (14 - 23 m/s) measured in squid giant axons..
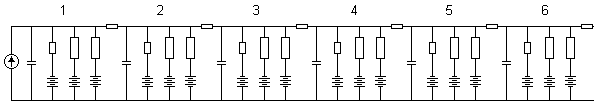
Fig. 10.15. The Lewis model that simulates the propagation of the action pulse.
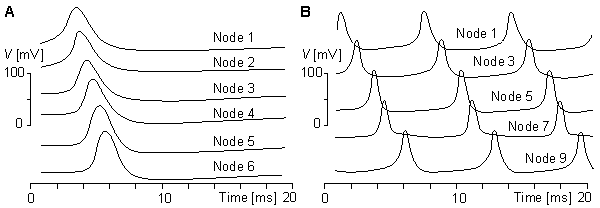
Fig. 10.16. Propagation of the action pulse in the Lewis model in
 (A) a six-unit chain and
(A) a six-unit chain and
 (B) a ten-unit ring.
(B) a ten-unit ring. Stefan Prange (1988, 1990) has developed an electronic neuron model that is realized with integrated circuit technology. The circuit includes one neuron with eight synapses. The chip area of the integrated circuit is 4.5 × 5 mm2. The array contains about 200 NPN and 100 PNP transistors, and about 200 of them are used. The circuit is fabricated with one metal layer with a line width of 12 µm. Because the model is realized with integrated circuit technology, it is easy to produce in large quantities, which is necessary for simulating neural networks. These experiments, however, have not yet been made with this model.
Stefan Prange (1988, 1990) has developed an electronic neuron model that is realized with integrated circuit technology. The circuit includes one neuron with eight synapses. The chip area of the integrated circuit is 4.5 × 5 mm2. The array contains about 200 NPN and 100 PNP transistors, and about 200 of them are used. The circuit is fabricated with one metal layer with a line width of 12 µm. Because the model is realized with integrated circuit technology, it is easy to produce in large quantities, which is necessary for simulating neural networks. These experiments, however, have not yet been made with this model.
 In 1991, Misha Mahowald and Rodney Douglas published an integrated circuit realization of electronic neuron model (Mahowald and Douglas, 1991). It was realized with complementary metal oxide semiconductor (CMOS) circuits using very large-scale integrated (VLSI) technology. Their model simulates very accurately the spikes of a neocortical neuron. The power dissipation of the circuit is 60 µW, and it occupies less than 0.1 mm2. The authors estimate that 100-200 such neurons could be fabricated on a 1 cm × 1 cm die.
In 1991, Misha Mahowald and Rodney Douglas published an integrated circuit realization of electronic neuron model (Mahowald and Douglas, 1991). It was realized with complementary metal oxide semiconductor (CMOS) circuits using very large-scale integrated (VLSI) technology. Their model simulates very accurately the spikes of a neocortical neuron. The power dissipation of the circuit is 60 µW, and it occupies less than 0.1 mm2. The authors estimate that 100-200 such neurons could be fabricated on a 1 cm × 1 cm die.


